Search
- Page Path
- HOME > Search
Original Articles
- Recent update of proton beam therapy for hepatocellular carcinoma: A systematic review and meta-analysis
- Sun Hyun Bae, Won Il Jang, Hanna Rahbek Mortensen, Britta Weber, Mi Sook Kim, Morten Høyer
- Received May 16, 2024 Accepted June 26, 2024 Published online July 4, 2024
- DOI: https://doi.org/10.17998/jlc.2024.06.26 [Accepted]
- 420 Views
- 16 Downloads
-
 Abstract
Abstract
 PDF
PDF - Backgrounds/Aims
Although access to proton beam therapy (PBT) is limited worldwide, its use for the treatment of hepatocellular carcinoma (HCC) is gradually increasing with the expansion of new facilities. Therefore, we conducted a systematic review and meta-analysis to investigate the updated evidence of PBT for HCC.
Methods
The MEDLINE, EMBASE, Cochrane Library, and Web of Science databases were systematically searched for studies that enrolled patients with liver-confined HCC that were treated with PBT for a cure up to February 2024.
Results
A total of 1858 HCC patients receiving PBT from 22 studies between 2004 and 2023 were selected for this meta-analysis. The median proportion of Child-Pugh class A was 86% (range: 41–100%), and the median tumor size was 3.6 cm (range: 1.2–9 cm). The median total dose ranged from 55 GyE to 76 GyE (median, 69 GyE). The pooled rates of 3- and 5-year local progression-free survival after PBT were 88% (95% confidence interval [CI], 85–91%) and 86% (95% CI, 82–90%), respectively. The pooled 3- and 5-year overall rates were 60% (95% CI, 54–66%) and 46% (95% CI, 38–54%), respectively. The pooled rates of grade 3 hepatic toxicity, classic radiation-induced liver disease (RILD), and non-classic RILD were 1%, 2%, and 1%, respectively.
Conclusions
The current study supports PBT for HCC and demonstrates favorable long-term survival and low hepatic toxicities compared with other published studies on other radiotherapy modalities. However, further studies are needed to identify the subgroups that will benefit from PBT.

- Re-assessing the diagnostic value of the enhancing “capsule” in hepatocellular carcinoma imaging
- Jae Seok Bae, Jeong Min Lee, Bo Yun Hur, Jeongin Yoo, Sae-Jin Park
- Received March 8, 2024 Accepted May 1, 2024 Published online May 8, 2024
- DOI: https://doi.org/10.17998/jlc.2024.05.01 [Accepted]

- 1,072 Views
- 19 Downloads
-
 Abstract
Abstract
 PDF
PDF - Background/Aims
The enhancing “capsule” (EC) in hepatocellular carcinoma (HCC) diagnosis has received varying degrees of recognition across major guidelines. This study aimed to assess the diagnostic utility of EC in HCC detection.
Methods
We retrospectively analyzed patients who underwent pre-surgical computed tomography (CT) and hepatobiliary agent-enhanced magnetic resonance imaging (HBA-MRI) between January 2016 and December 2019. A single hepatic tumor was confirmed based on the pathology of each patient. Three radiologists independently reviewed the images according to the Liver Imaging Reporting and Data System (LIRADS) v2018 criteria and reached a consensus. Interobserver agreement for EC before reaching a consensus was quantified using Fleiss κ statistics. The impact of EC on the LI-RADS classification was assessed by comparing the positive predictive values for HCC detection in the presence and absence of EC.
Results
In total, 237 patients (median age, 60 years; 184 men) with 237 observations were included. The interobserver agreement for EC detection was notably low for CT (κ=0.169) and HBA-MRI (κ=0.138). The presence of EC did not significantly alter the positive predictive value for HCC detection in LI-RADS category 5 observations on CT (94.1% [80/85] vs. 94.6% [88/93], P=0.886) or HBA-MRI (95.7% [88/92] vs. 90.6% [77/85], P=0.178).
Conclusions
The diagnostic value of EC in HCC diagnosis remains questionable, given its poor interobserver agreement and negligible impact on positive predictive values for HCC detection. This study challenges the emphasis on EC in certain diagnostic guidelines and suggests the need to re-evaluate its role in HCC imaging.

Review Articles
- Advancing Korean nationwide registry for hepatocellular carcinoma: a systematic sampling approach utilizing the Korea Central Cancer Registry database
- Bo Hyun Kim, E Hwa Yun, Jeong-Hoon Lee, Geun Hong, Jun Yong Park, Ju Hyun Shim, Eunyang Kim, Hyun-Joo Kong, Kyu-Won Jung, Young-Suk Lim
- J Liver Cancer. 2024;24(1):57-61. Published online March 26, 2024
- DOI: https://doi.org/10.17998/jlc.2024.03.03
- 1,021 Views
- 38 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
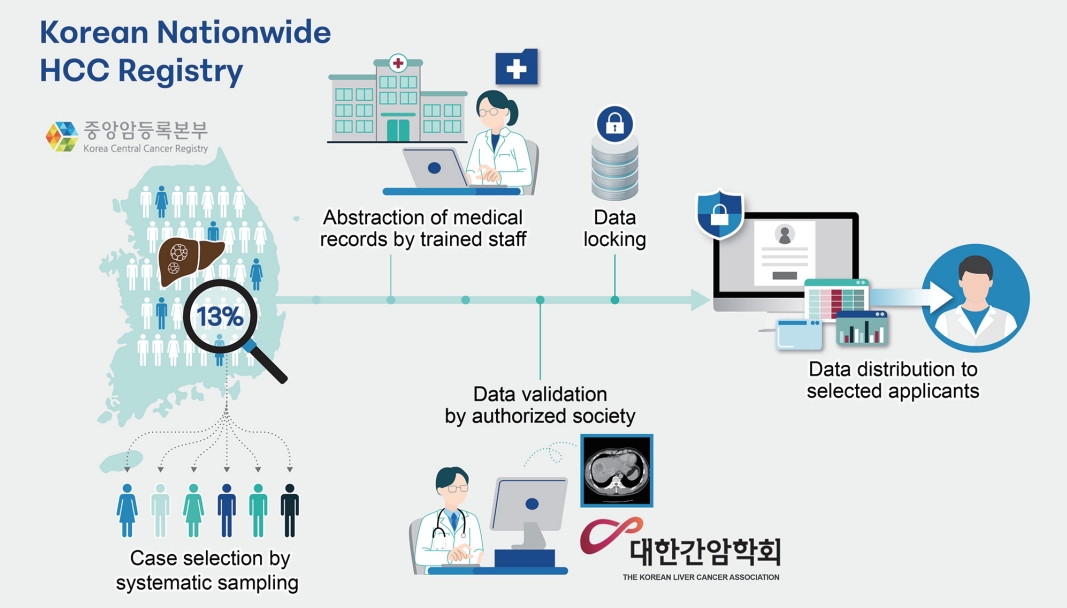
Supplementary Material - Hepatocellular carcinoma (HCC) presents a substantial public health challenge in South Korea as evidenced by 10,565 new cases annually (incidence rate of 30 per 100,000 individuals), in 2020. Cancer registries play a crucial role in gathering data on incidence, disease attributes, etiology, treatment modalities, outcomes, and informing health policies. The effectiveness of a registry depends on the completeness and accuracy of data. Established in 1999 by the Ministry of Health and Welfare, the Korea Central Cancer Registry (KCCR) is a comprehensive, legally mandated, nationwide registry that captures nearly all incidence and survival data for major cancers, including HCC, in Korea. However, detailed information on cancer staging, specific characteristics, and treatments is lacking. To address this gap, the KCCR, in partnership with the Korean Liver Cancer Association (KLCA), has implemented a systematic approach to collect detailed data on HCC since 2010. This involved random sampling of 10-15% of all new HCC cases diagnosed since 2003. The registry process encompassed four stages: random case selection, meticulous data extraction by trained personnel, expert validation, anonymization of personal data, and data dissemination for research purposes. This random sampling strategy mitigates the biases associated with voluntary reporting and aligns with stringent privacy regulations. This innovative approach positions the KCCR and KLCA as foundations for advancing cancer control and shaping health policies in South Korea.
-
Citations
Citations to this article as recorded by- Association of modifiable metabolic risk factors and lifestyle with all-cause mortality in patients with hepatocellular carcinoma
Hwi Young Kim, Hye Ah Lee, Pompilia Radu, Jean-François Dufour
Scientific Reports.2024;[Epub] CrossRef
- Association of modifiable metabolic risk factors and lifestyle with all-cause mortality in patients with hepatocellular carcinoma

- Changing etiology and epidemiology of hepatocellular carcinoma: Asia and worldwide
- Do Young Kim
- J Liver Cancer. 2024;24(1):62-70. Published online March 25, 2024
- DOI: https://doi.org/10.17998/jlc.2024.03.13
- 2,792 Views
- 185 Downloads
- 2 Citations
-
 Abstract
Abstract
 PDF
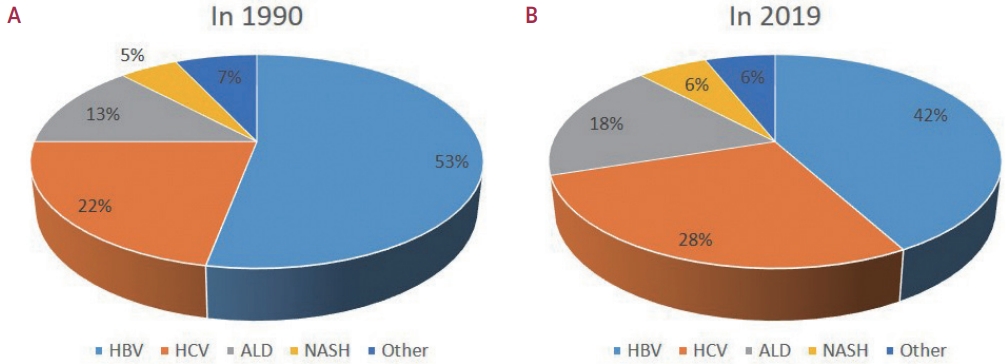
PDF - Approximately 80% of hepatocellular carcinoma (HCC) cases arise in sub-Saharan Africa and Eastern Asia, following a similarly high prevalence of chronic hepatitis B virus (HBV) carriers in these regions. The etiology and epidemiology of HCC have recently changed worldwide. Although HBV infection is the main contributor to HCC development, a slow but continuous decline in HBV infection rates has been reported since 1990. Owing to the widespread use of direct-acting antivirals, the incidence of hepatitis C virus-related HCC has remarkably decreased in Japan and European countries. In Korea, Taiwan, and Singapore, the incidence of HBV-related HCC has significantly decreased owing to vaccination against HBV. Globally, while HBV accounted for more than half of HCCs in 1990, this had decreased to 42% in 2019. In contrast, the proportion of patients with alcoholic- and nonalcoholic steatohepatitis (NASH) increased from 13% to 18% and from 5% to 6%, respectively. NASH-related HCC has characteristics that differ from those of virus-associated HCC. Compared with other etiologies, patients with NASHassociated HCC are older, have a higher body mass index, and have higher rates of type 2 diabetes mellitus, hypertension, hyperlipidemia, and cardiovascular disease. Nonalcoholic fatty liver disease (NAFLD)-associated HCC is also known to develop in the absence of cirrhosis, unlike alcohol-related and autoimmune liver diseases. Because patients with NAFLD usually have diabetes or obesity, surveying this population is challenging. Optimal selection of the target population and surveillance tools among patients with NAFLD needs to be determined.
-
Citations
Citations to this article as recorded by- Immunotherapy as a Complement to Surgical Management of Hepatocellular Carcinoma
Susan J. Kim, Kaelyn C. Cummins, Allan Tsung
Cancers.2024; 16(10): 1852. CrossRef - Inflammatory Response in the Pathogenesis and Treatment of Hepatocellular Carcinoma: A Double-Edged Weapon
Linda Galasso, Lucia Cerrito, Valeria Maccauro, Fabrizio Termite, Irene Mignini, Giorgio Esposto, Raffaele Borriello, Maria Elena Ainora, Antonio Gasbarrini, Maria Assunta Zocco
International Journal of Molecular Sciences.2024; 25(13): 7191. CrossRef
- Immunotherapy as a Complement to Surgical Management of Hepatocellular Carcinoma

- Multidisciplinary approach for hepatocellular carcinoma patients: current evidence and future perspectives
- Joo Hyun Oh, Dong Hyun Sinn
- J Liver Cancer. 2024;24(1):47-56. Published online March 25, 2024
- DOI: https://doi.org/10.17998/jlc.2024.02.27

- 1,441 Views
- 89 Downloads
-
 Abstract
Abstract
 PDF
PDF - Management of hepatocellular carcinoma (HCC) is challenging due to the complex relationship between underlying liver disease, tumor burden, and liver function. HCC is also notorious for its high recurrence rate even after curative treatment for early-stage tumor. Liver transplantation can substantially alter patient prognosis, but donor availability varies by each patient which further complicates treatment decision. Recent advancements in HCC treatments have introduced numerous potentially efficacious treatment modalities. However, high level evidence comparing the risks and benefits of these options is limited. In this complex situation, multidisciplinary approach or multidisciplinary team care has been suggested as a valuable strategy to help cope with escalating complexity in HCC management. Multidisciplinary approach involves collaboration among medical and health care professionals from various academic disciplines to provide comprehensive care. Although evidence suggests that multidisciplinary care can enhance outcomes of HCC patients, robust data from randomized controlled trials are currently lacking. Moreover, the implementation of a multidisciplinary approach necessitates increased medical resources compared to conventional cancer care. This review summarizes the current evidence on the role of multidisciplinary approach in HCC management and explores potential future directions.

- Current perspectives on radiotherapy in hepatocellular carcinoma management: a comprehensive review
- Dowook Kim, Jun-Sang Kim
- J Liver Cancer. 2024;24(1):33-46. Published online March 25, 2024
- DOI: https://doi.org/10.17998/jlc.2024.02.26
- 1,554 Views
- 100 Downloads
-
 Abstract
Abstract
 PDF
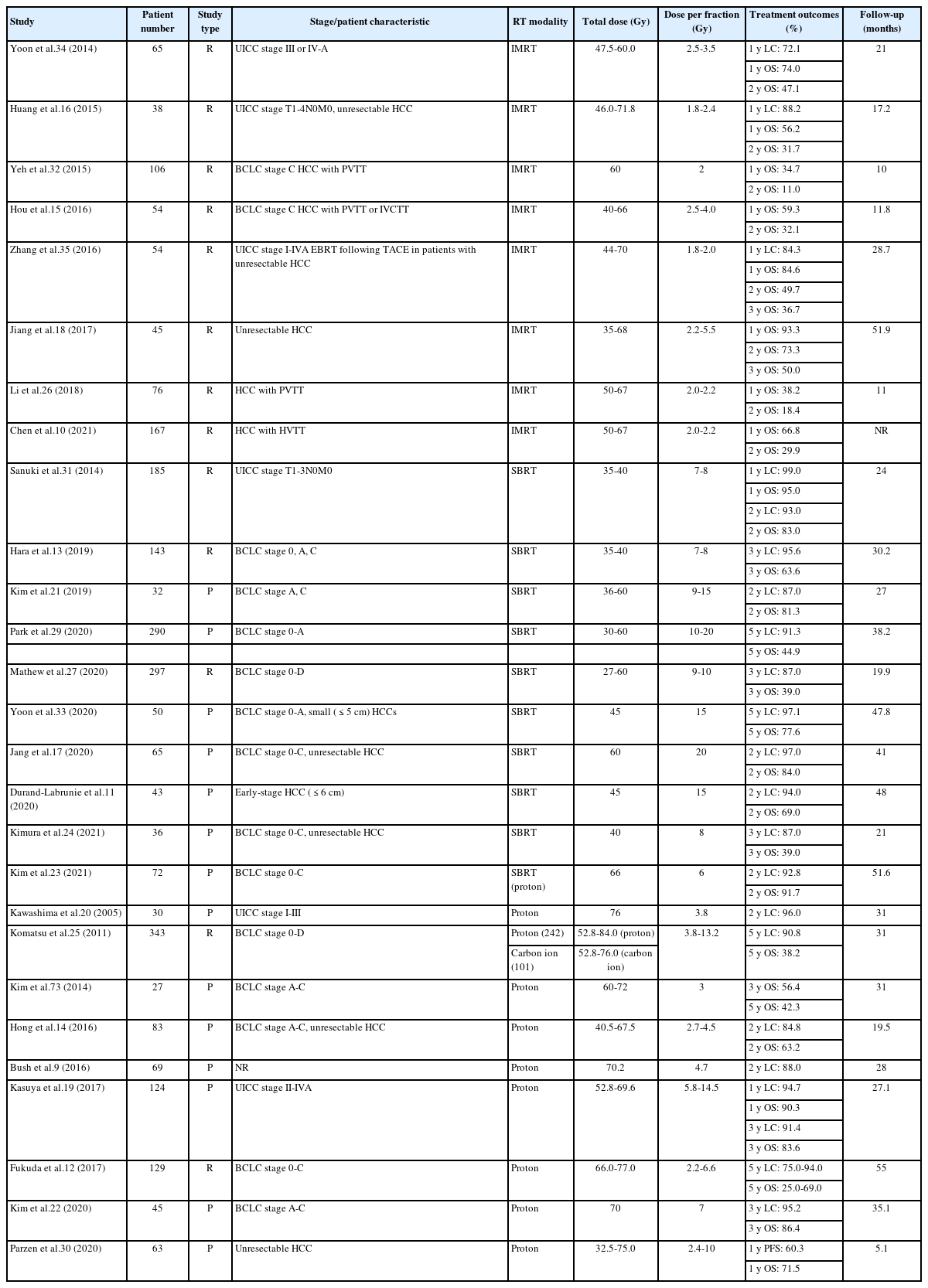
PDF - This review examines the transformative role of external beam radiotherapy (EBRT) in managing hepatocellular carcinoma (HCC), spotlighting the progression from traditional EBRT techniques to advanced modalities like intensity-modulated radiotherapy (RT), stereotactic body RT (SBRT), and innovative particle therapy, including proton beam therapy and carbon ion RT. These advancements have significantly improved the precision and efficacy of RT, marking a paradigm shift in the multimodal management of HCC, particularly in addressing complex cases and enhancing local tumor control. The review underscores the synergistic potential of integrating RT with other treatments like transarterial chemoembolization, systemic therapies such as sorafenib, and emerging immunotherapies, illustrating enhanced survival and disease control outcomes. The efficacy of RT is addressed for challenging conditions, including advanced HCC with macrovascular invasion, and RT modalities, like SBRT, are compared against traditional treatments like radiofrequency ablation for early-stage HCC. Additionally, the review accentuates the encouraging outcomes of particle therapy in enhancing local control and survival rates, minimizing treatment-related toxicity, and advocating for continued research and clinical trials. In conclusion, the integration of RT into multimodal HCC treatment strategies, coupled with the emergence of particle therapy, is crucial for advancing oncologic management, emphasizing the need for relentless innovation and personalized treatment approaches.

- Intermediate-stage hepatocellular carcinoma: refining substaging or shifting paradigm?
- Bernardo Stefanini, Luca Ielasi, Dante Pio Pallotta, Sofia Penazza, Mariarosaria Marseglia, Fabio Piscaglia
- J Liver Cancer. 2024;24(1):23-32. Published online March 12, 2024
- DOI: https://doi.org/10.17998/jlc.2024.02.21
- 1,525 Views
- 94 Downloads
-
 Abstract
Abstract
 PDF
PDF - This review explores the evolution of cancer staging, focusing on intermediate hepatocellular carcinoma (HCC), and the challenges faced by physicians. The Barcelona Clinic Liver Cancer (BCLC) staging system, introduced in 1999, was designed to address the limitations associated with providing accurate prognostic information for HCC and allocating specific treatments, to avoid overtreatment. However, criticism has emerged, particularly regarding the intermediate stage of HCC (BCLC-B) and its heterogeneous patient population. To overcome this limitation, various subclassification systems, such as the Bolondi and Kinki criteria, have been proposed. These systems are aimed at refining categorizations within the intermediate stage and have demonstrated varying degrees of success in predicting outcomes through external validation. This study discusses the shift in treatment paradigms, emphasizing the need for a more personalized approach rather than strictly adhering to cancer stages, without dismissing the relevance of staging systems. It assesses the available treatment options for intermediate-stage HCC, highlighting the importance of considering surgical and nonsurgical options alongside transarterial chemoembolization for optimal outcomes. In conclusion, the text advocates for a paradigm shift in staging systems prioritizing treatment suitability over cancer stage. This reflects the evolving landscape of HCC management, where a multidisciplinary approach is crucial for tailoring treatments to individual patients, ultimately aiming to improve overall survival.

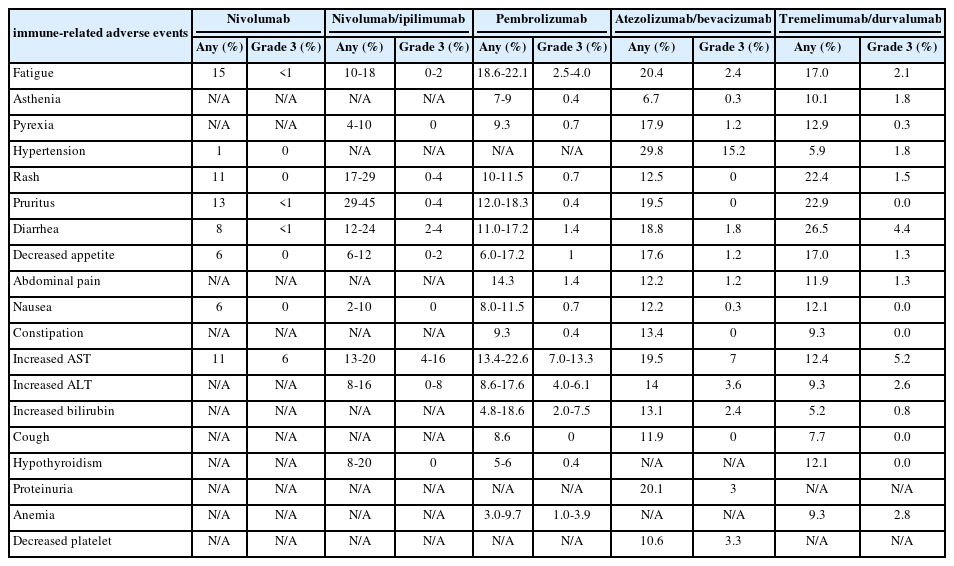
- Complications of immunotherapy in advanced hepatocellular carcinoma
- Young-Gi Song, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
- J Liver Cancer. 2024;24(1):9-16. Published online November 29, 2023
- DOI: https://doi.org/10.17998/jlc.2023.11.21
- 2,113 Views
- 122 Downloads
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF - Immune checkpoint inhibitors (ICIs) are highly effective in cancer treatment. However, the risks associated with the treatment must be carefully balanced against the therapeutic benefits. Immune-related adverse events (irAEs) are generally unpredictable and may persist over an extended period. In this review, we analyzed common irAEs reported in highly cited original articles and systematic reviews. The prevalent adverse reactions include fatigue, pyrexia, rash, pruritus, diarrhea, decreased appetite, nausea, abdominal pain, constipation, hepatitis, and hypothyroidism. Therefore, it is crucial to conduct evaluations not only of gastrointestinal organs but also of cardiac, neurologic, endocrine (including the frequently affected thyroid), and ophthalmic systems before commencing ICIs. This review further explores commonly reported types of irAEs, specific irAEs associated with each ICI agent, rare yet potentially fatal irAEs, and available treatment options for managing them.
-
Citations
Citations to this article as recorded by- Intrahepatic IgA complex induces polarization of cancer-associated fibroblasts to matrix phenotypes in the tumor microenvironment of HCC
Jong Geun Park, Pu Reun Roh, Min Woo Kang, Sung Woo Cho, Suhyun Hwangbo, Hae Deok Jung, Hyun Uk Kim, Ji Hoon Kim, Jae-Sung Yoo, Ji Won Han, Jeong Won Jang, Jong Young Choi, Seung Kew Yoon, Young Kyoung You, Ho Joong Choi, Jae Yong Ryu, Pil Soo Sung
Hepatology.2024;[Epub] CrossRef - Risk of Bleeding in Hepatocellular Carcinoma Patients Treated with Atezolizumab/Bevacizumab: A Systematic Review and Meta-Analysis
Young-Gi Song, Kyeong-Min Yeom, Eun Ae Jung, Sang Gyune Kim, Young Seok Kim, Jeong-Ju Yoo
Liver Cancer.2024; : 1. CrossRef
- Intrahepatic IgA complex induces polarization of cancer-associated fibroblasts to matrix phenotypes in the tumor microenvironment of HCC

- A multidisciplinary approach with immunotherapies for advanced hepatocellular carcinoma
- Yu Rim Lee
- J Liver Cancer. 2023;23(2):316-329. Published online September 22, 2023
- DOI: https://doi.org/10.17998/jlc.2023.09.04

- 2,030 Views
- 115 Downloads
- 4 Citations
-
 Abstract
Abstract
 PDF
PDF - Hepatocellular carcinoma (HCC) is a highly aggressive disease that is usually diagnosed at an advanced stage. Advanced HCC has limited treatment options and often has a poor prognosis. For the past decade, tyrosine kinase inhibitors have been the only treatments approved for advanced HCC that have shown overall survival (OS) benefits; however, but their clinical efficacy has been limited. Recent trials have demonstrated promising advancements in survival outcomes through immunotherapy-based treatments, such as combinations of immune checkpoint inhibitors (ICIs) with other ICIs, antiangiogenic drugs, and locoregional therapies. The atezolizumab-bevacizumab and durvalumab-tremelimumab (STRIDE) regimen has significantly improved survival rates as a first-line treatment and has become the new standard of care. Therefore, combined treatments for advanced HCC can result in better treatment outcomes owing to their synergistic effects, which requires a multidisciplinary approach. Ongoing studies are examining other therapeutic innovations that can improve disease control and OS rates. Despite improvements in the treatment of advanced HCC, further studies on the optimal treatment selection and sequences, biomarker identification, combination approaches with other therapies, and development of novel immunotherapy agents are required. This review presents the current treatment options and clinical data of the ICI-based combination immunotherapies for advanced HCC from a multidisciplinary perspective.
-
Citations
Citations to this article as recorded by- Reduced-Dose or Discontinuation of Bevacizumab Might Be Considered after Variceal Bleeding in Patients with Hepatocellular Carcinoma Receiving Atezolizumab/Bevacizumab: Case Reports
Kyeong-Min Yeom, Young-Gi Song, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
Medicina.2024; 60(1): 157. CrossRef - Hepatocellular Carcinoma: Advances in Systemic Therapy
Insija Ilyas Selene, Merve Ozen, Reema A. Patel
Seminars in Interventional Radiology.2024; 41(01): 056. CrossRef - Fatal intratumoral hemorrhage in a patient with hepatocellular carcinoma following successful treatment with atezolizumab/bevacizumab: A case report
Kyeong-Hoon Park, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
World Journal of Clinical Cases.2024; 12(22): 5177. CrossRef - Unravelling infiltrating T‐cell heterogeneity in kidney renal clear cell carcinoma: Integrative single‐cell and spatial transcriptomic profiling
Haiqing Chen, Haoyuan Zuo, Jinbang Huang, Jie Liu, Lai Jiang, Chenglu Jiang, Shengke Zhang, Qingwen Hu, Haotian Lai, Bangchao Yin, Guanhu Yang, Gang Mai, Bo Li, Hao Chi
Journal of Cellular and Molecular Medicine.2024;[Epub] CrossRef
- Reduced-Dose or Discontinuation of Bevacizumab Might Be Considered after Variceal Bleeding in Patients with Hepatocellular Carcinoma Receiving Atezolizumab/Bevacizumab: Case Reports

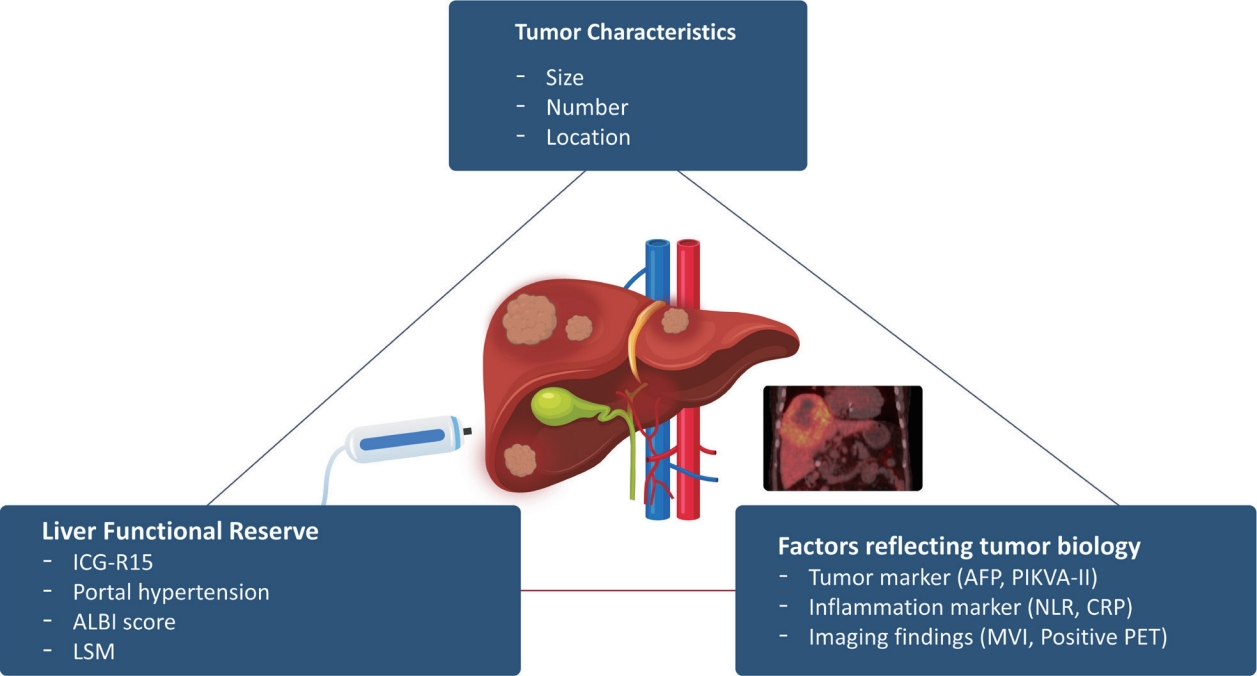
- Management of early-stage hepatocellular carcinoma: challenges and strategies for optimal outcomes
- Jae Hyun Yoon, Sung Kyu Choi
- J Liver Cancer. 2023;23(2):300-315. Published online September 21, 2023
- DOI: https://doi.org/10.17998/jlc.2023.08.27
- 2,905 Views
- 167 Downloads
- 6 Citations
-
 Abstract
Abstract
 PDF
PDF - Although hepatocellular carcinoma (HCC) is associated with a poor prognosis, management of early-stage HCC is often successful with highly efficacious treatment modalities such as liver transplantation, surgical resection, and radiofrequency ablation. However, unfavorable clinical outcomes have been observed under certain circumstances, even after efficient treatment. Factors that predict unsuitable results after treatment include tumor markers, inflammatory markers, imaging findings reflecting tumor biology, specific outcome indicators for each treatment modality, liver functional reserve, and the technical feasibility of the treatment modalities. Various strategies may overcome these challenges, including the application of reinforced treatment indication criteria with predictive markers reflecting tumor biology, compensation for technical issues with up-to-date technologies, modification of treatment modalities, downstaging with locoregional therapies (such as transarterial chemotherapy or radiotherapy), and recently introduced combination immunotherapies. In this review, we discuss the challenges to achieving optimal outcomes in the management of early-stage HCC and suggest strategies to overcome these obstacles.
-
Citations
Citations to this article as recorded by- Diosgenin potentiates the anticancer effect of doxorubicin and volasertib via regulating polo-like kinase 1 and triggering apoptosis in hepatocellular carcinoma cells
Eman H. Yousef, Mohamed E. El-Mesery, Maha R. Habeeb, Laila A. Eissa
Naunyn-Schmiedeberg's Archives of Pharmacology.2024; 397(7): 4883. CrossRef - Comparison of Surgical Resection and Radiofrequency Ablation in Elderly Patients with Hepatocellular Carcinoma
Jun Il Kim, Jayoun Lee, Gi Hong Choi, Min Woo Lee, Dong Ah Park, Jeong-Ju Yoo
Digestive Diseases and Sciences.2024; 69(3): 1055. CrossRef - Radiofrequency for hepatocellular carcinoma larger than 3 cm: potential for applications in daily practice
Ji Hoon Kim, Pil Soo Sung
Journal of Liver Cancer.2024; 24(1): 1. CrossRef - Hepatocellular carcinoma outcomes and potential implications for surveillance in elderly patients
Aryoung Kim, Goeun Park, Myung Ji Goh, Byeong Geun Song, Wonseok Kang, Geum-Youn Gwak, Yong-Han Paik, Moon Seok Choi, Joon Hyeok Lee, Dong Hyun Sinn
Scientific Reports.2024;[Epub] CrossRef - Trends in alcohol use and alcoholic liver disease in South Korea: a nationwide cohort study
Jeong-Ju Yoo, Dong Hyeon Lee, Young Chang, Hoongil Jo, Young Youn Cho, Sangheun Lee, Log Young Kim, Jae Young Jang
BMC Public Health.2024;[Epub] CrossRef - Efficacy of Transarterial Chemoembolization (TACE) for Early-Stage Hepatocellular Carcinoma
Moonhyung Lee, Hyun Phil Shin
Medicina.2023; 59(12): 2174. CrossRef
- Diosgenin potentiates the anticancer effect of doxorubicin and volasertib via regulating polo-like kinase 1 and triggering apoptosis in hepatocellular carcinoma cells

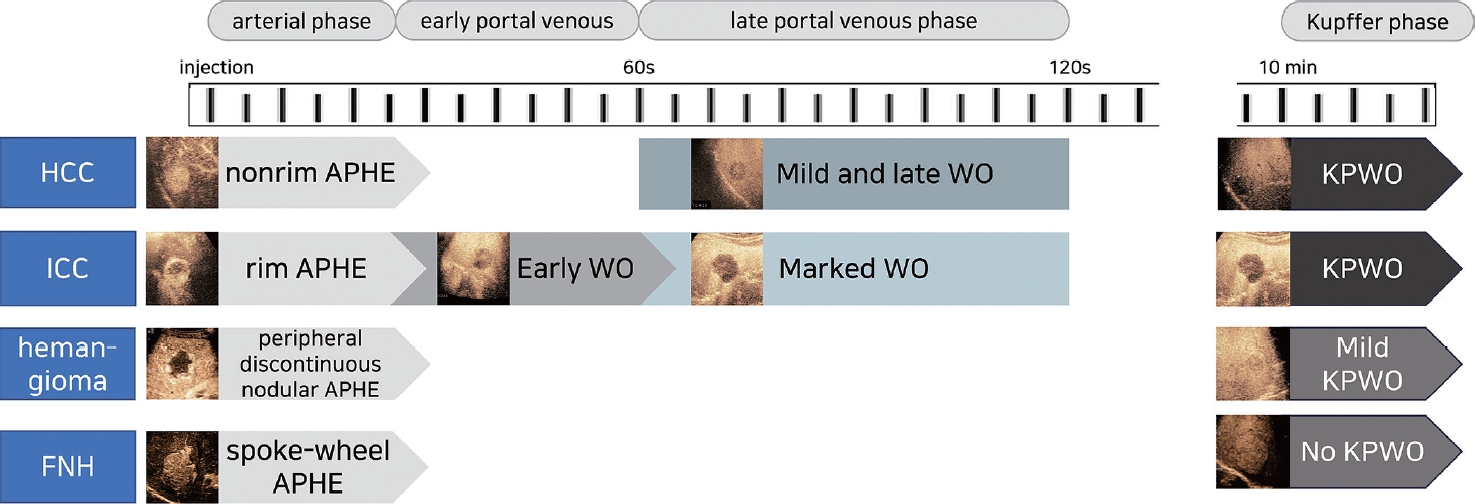
- Diagnosis of hepatocellular carcinoma using Sonazoid: a comprehensive review
- Woo Kyoung Jeong
- J Liver Cancer. 2023;23(2):272-283. Published online September 19, 2023
- DOI: https://doi.org/10.17998/jlc.2023.08.25
- 1,568 Views
- 99 Downloads
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF - Sonazoid contrast-enhanced ultrasonography (CEUS) is a promising technique for the detection and diagnosis of focal liver lesions, particularly hepatocellular carcinoma (HCC). Recently, a collaborative effort between the Korean Society of Radiology and Korean Society of Abdominal Radiology resulted in the publication of guidelines for diagnosing HCC using Sonazoid CEUS. These guidelines propose specific criteria for identifying HCC based on the imaging characteristics observed during Sonazoid CEUS. The suggested diagnostic criteria include nonrim arterial phase hyperenhancement, and the presence of late and mild washout, or Kupffer phase washout under the premise that the early or marked washout should not occur during the portal venous phase. These criteria aim to improve the accuracy of HCC diagnosis using Sonazoid CEUS. This review offers a comprehensive overview of Sonazoid CEUS in the context of HCC diagnosis. It covers the fundamental principles of Sonazoid CEUS and its clinical applications, and introduces the recently published guidelines. By providing a summary of this emerging technique, this review contributes to a better understanding of the potential role of Sonazoid CEUS for diagnosing HCC.
-
Citations
Citations to this article as recorded by- The Diagnostic Impact of Contrast-Enhanced Computed Tomography (CECT) in Evaluating Lymph Node Involvement in Colorectal Cancer: A Comprehensive Review
Akash Inamdar, Raju K Shinde
Cureus.2024;[Epub] CrossRef - Sonazoid contrast-enhanced ultrasonography for the diagnosis of hepatocellular carcinoma: strengths and shortcomings
Sung Won Lee, Min Kyu Kang, Xiang Zhang
Journal of Liver Cancer.2023; 23(2): 238. CrossRef
- The Diagnostic Impact of Contrast-Enhanced Computed Tomography (CECT) in Evaluating Lymph Node Involvement in Colorectal Cancer: A Comprehensive Review

- Imaging prognostication and tumor biology in hepatocellular carcinoma
- Diana Kadi, Marilyn F. Yamamoto, Emily C. Lerner, Hanyu Jiang, Kathryn J. Fowler, Mustafa R. Bashir
- J Liver Cancer. 2023;23(2):284-299. Published online September 15, 2023
- DOI: https://doi.org/10.17998/jlc.2023.08.29

- 2,534 Views
- 122 Downloads
-
 Abstract
Abstract
 PDF
PDF - Hepatocellular carcinoma (HCC) is the most common primary liver malignancy, and represents a significant global health burden with rising incidence rates, despite a more thorough understanding of the etiology and biology of HCC, as well as advancements in diagnosis and treatment modalities. According to emerging evidence, imaging features related to tumor aggressiveness can offer relevant prognostic information, hence validation of imaging prognostic features may allow for better noninvasive outcomes prediction and inform the selection of tailored therapies, ultimately improving survival outcomes for patients with HCC.

Original Articles
- Clinical outcome of surgical resection for multifocal T2-T3 hepatocellular carcinoma up to 3 nodules: a comparative analysis with a single nodule
- Sehyeon Yu, Hye-Sung Jo, Young-Dong Yu, Yoo jin Choi, Dong-Sik Kim
- J Liver Cancer. 2023;23(2):377-388. Published online September 15, 2023
- DOI: https://doi.org/10.17998/jlc.2023.08.24

- 827 Views
- 42 Downloads
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background/Aims
Although the Barcelona Clinic Liver Cancer staging system seems to underestimate the impact of curative-intent surgical resection for multifocal hepatocellular carcinoma (HCC), recent studies have indicated favorable results for the surgical resection of multiple HCC. This study aimed to assess clinical outcomes and feasibility of surgical resection for multifocal HCC with up to three nodules compared with single tumor cases.
Methods
Patients who underwent surgical resection for HCC with up to three nodules between 2009 and 2020 were included, and those with the American Joint Committee on Cancer (AJCC) 8th edition, T1 and T4 stages were excluded to reduce differences in disease distribution and severity. Finally, 81 and 52 patients were included in the single and multiple treatment groups, respectively. Short- and long-term outcomes including recurrence-free survival (RFS) and overall survival (OS), were evaluated.
Results
All patients were classified as Child-Pugh class A. RFS and OS were not significantly different between the two groups (P=0.176 and P=0.966, respectively). Multivariate analysis revealed that transfusion and intrahepatic metastasis were significantly associated with recurrence (P=0.046 and P=0.005, respectively). Additionally, intrahepatic metastasis was significantly associated with OS (hazard ratio, 1.989; 95% confidence interval, 1.040-3.802; P=0.038).
Conclusions
Since there was no significant difference in survival between the single and multiple groups among patients with AJCC 8th stage T2 and T3, surgical resection with curative intent could be considered with acceptable long-term survival for selected patients with multiple HCC of up to three nodules.

- Nomogram for predicting overall survival in patients with large (>5 cm) hepatocellular carcinoma based on real-world practice
- Nalee Kim, Jeong Il Yu, Hee Chul Park, Jung Yong Hong, Ho Yeong Lim, Myung Ji Goh, Yong-Han Paik
- J Liver Cancer. 2023;23(2):350-361. Published online September 6, 2023
- DOI: https://doi.org/10.17998/jlc.2023.08.10

- 1,184 Views
- 54 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background/Aim
Patients with large (>5 cm) hepatocellular carcinoma (HCC) have limited treatment options, thus necessitating the identification of prognostic factors and the development of predictive tools. This study aimed to identify prognostic factors and to construct a nomogram to predict survival outcomes in patients with large HCC.
Methods
A cohort of 438 patients, who were diagnosed with large HCC at a tertiary hospital between 2015 and 2018, was analyzed. Cox proportional hazards models were used to identify key prognosticators of overall survival (OS), and an independent set of prognostic factors was used to develop a nomogram. The discrimination and calibration abilities of the nomogram were assessed and internal validation was performed using cross-validation and bootstrapping methods.
Results
During a median follow-up of 9.3 months, the median OS was 9.9 months, and the 1-year OS rate was 43.9%. Multivariable Cox regression analysis revealed that performance status, modified albumin-bilirubin grade, tumor size, extent of portal vein tumor thrombosis, and initial treatment significantly affected OS. The newly developed nomogram incorporating these variables demonstrated favorable accuracy (Harrell’s concordance index, 0.807).
Conclusions
The newly developed nomogram facilitated the estimation of individual survival outcomes in patients with large HCC, providing an acceptable level of accuracy. -
Citations
Citations to this article as recorded by- Prognostic Role of Basal Serum Alpha-Fetoprotein in Patients with Hepatocellular Carcinoma Suitable for Curative Treatment
Stefano Mazza, Chiara Frigerio, Daniele Alfieri, Aurelio Mauro, Francesca Torello Viera, Davide Scalvini, Chiara Barteselli, Carmelo Sgarlata, Letizia Veronese, Marco Bardone, Laura Rovedatti, Simona Agazzi, Elena Strada, Lodovica Pozzi, Marcello Maestri,
Medicina.2024; 60(5): 692. CrossRef
- Prognostic Role of Basal Serum Alpha-Fetoprotein in Patients with Hepatocellular Carcinoma Suitable for Curative Treatment

Review Article
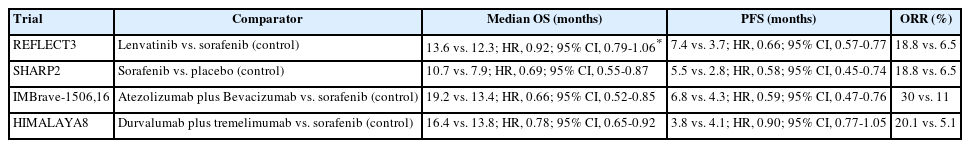
- The role of lenvatinib in the era of immunotherapy of hepatocellular carcinoma
- Matthew Man Pok Lee, Landon Long Chan, Stephen Lam Chan
- J Liver Cancer. 2023;23(2):262-271. Published online August 17, 2023
- DOI: https://doi.org/10.17998/jlc.2023.07.17
- 3,531 Views
- 281 Downloads
- 5 Citations
-
 Abstract
Abstract
 PDF
PDF - Hepatocellular carcinoma (HCC) frequently presents as advanced stage with poor prognosis and high mortality. Systemic treatment is the treatment of choice for advanced disease. In 2007, the first multi-kinase inhibitor (MKI) sorafenib was approved and shown to modestly prolong overall survival (OS). The progress of systemic therapy has been slow afterwards until 2018 when lenvatinib, another MKI, was shown to be non-inferior to sorafenib on median OS as the first-line therapy for HCC. Since then, remarkable progress has been achieved on the treatment of advanced HCC, including the development of second-line targeted treatment, including regorafenib, cabozantinib and ramucirumab from 2017 to 2019. A growing focus has been placed on immune checkpoint inhibitors (ICIs) targeting programmed cell death-1 (PD-1), its ligand PD-L1, and cytotoxic T-lymphocyte-associated protein 4. These ICIs have proven their potency in treating HCC as both initial and subsequent line of therapy. At present, both regimens of atezolizumab combined with bevacizumab, as well as the combination of tremelimumab and durvalumab, are recommended as the first-line treatments based on positive phase III clinical trials. With the advancement of ICIs, it is anticipated that the role of MKIs in the treatment of HCC will evolve. In this article, lenvatinib, one of the most commonly used MKIs in HCC, is chosen to be reviewed.
-
Citations
Citations to this article as recorded by- Reduced-Dose or Discontinuation of Bevacizumab Might Be Considered after Variceal Bleeding in Patients with Hepatocellular Carcinoma Receiving Atezolizumab/Bevacizumab: Case Reports
Kyeong-Min Yeom, Young-Gi Song, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
Medicina.2024; 60(1): 157. CrossRef - The Position of Multikinase Inhibitors in the Era of Immune-Checkpoint Inhibitors for Hepatocellular Carcinoma
Beom Kyung Kim
Gut and Liver.2024; 18(1): 3. CrossRef - Fatal intratumoral hemorrhage in a patient with hepatocellular carcinoma following successful treatment with atezolizumab/bevacizumab: A case report
Kyeong-Hoon Park, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
World Journal of Clinical Cases.2024; 12(22): 5177. CrossRef - Small molecule tyrosine kinase inhibitors approved for systemic therapy of advanced hepatocellular carcinoma: recent advances and future perspectives
Jianzhong Liu, Shuai Xia, Baoyi Zhang, Dina Mostafa Mohammed, Xiangliang Yang, Yanhong Zhu, Xinnong Jiang
Discover Oncology.2024;[Epub] CrossRef - Consistent efficacy of hepatic artery infusion chemotherapy irrespective of PD‑L1 positivity in unresectable hepatocellular carcinoma
Ji Kim, Young Kim, Hee-Chul Nam, Chang-Wook Kim, Jae-Sung Yoo, Ji Han, Jeong Jang, Jong Choi, Seung Yoon, Ho Jong Chun, Jung Oh, Suho Kim, Sung Lee, Pil Sung
Oncology Letters.2024;[Epub] CrossRef
- Reduced-Dose or Discontinuation of Bevacizumab Might Be Considered after Variceal Bleeding in Patients with Hepatocellular Carcinoma Receiving Atezolizumab/Bevacizumab: Case Reports


 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION

 First
First Prev
Prev



 Follow JLC on Twitter
Follow JLC on Twitter