Articles
- Page Path
- HOME > J Liver Cancer > Volume 23(2); 2023 > Article
-
Review Article
Management of early-stage hepatocellular carcinoma: challenges and strategies for optimal outcomes -
Jae Hyun Yoon
 , Sung Kyu Choi
, Sung Kyu Choi
-
Journal of Liver Cancer 2023;23(2):300-315.
DOI: https://doi.org/10.17998/jlc.2023.08.27
Published online: September 21, 2023
Department of Gastroenterology and hepatology, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea
-
Corresponding author: Sung Kyu Choi, Department of Gastroenterology and hepatology, Chonnam National University Hospital, Chonnam National University Medical School, 160 Baekseo-ro, Dong-gu, Gwangju 61469, Korea
Tel. +82-62-220-6216, Fax. +82-62-220-8578 E-mail: choisk@jnu.ac.kr
© 2023 The Korean Liver Cancer Association.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,907 Views
- 167 Downloads
- 6 Citations
Abstract
- Although hepatocellular carcinoma (HCC) is associated with a poor prognosis, management of early-stage HCC is often successful with highly efficacious treatment modalities such as liver transplantation, surgical resection, and radiofrequency ablation. However, unfavorable clinical outcomes have been observed under certain circumstances, even after efficient treatment. Factors that predict unsuitable results after treatment include tumor markers, inflammatory markers, imaging findings reflecting tumor biology, specific outcome indicators for each treatment modality, liver functional reserve, and the technical feasibility of the treatment modalities. Various strategies may overcome these challenges, including the application of reinforced treatment indication criteria with predictive markers reflecting tumor biology, compensation for technical issues with up-to-date technologies, modification of treatment modalities, downstaging with locoregional therapies (such as transarterial chemotherapy or radiotherapy), and recently introduced combination immunotherapies. In this review, we discuss the challenges to achieving optimal outcomes in the management of early-stage HCC and suggest strategies to overcome these obstacles.
- Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related mortality globally, and seventh in terms of cancer incidence in South Korea. Although the overall clinical course and prognosis of HCC remain unsatisfactory, treatment is effective in select patients with early-stage HCC, potentially resulting in excellent recurrence-free and overall survival (OS) outcomes. Therefore, diagnosis of early-stage HCC and timely treatment are of utmost importance.
- The criteria for early-stage HCC vary according to the staging system. The Barcelona Clinic Liver Cancer (BCLC) system, one of the most widely used HCC staging systems, was updated in 2022.1 It defines early HCC into two groups: very early-stage HCC, which includes single HCC ≤2 cm with preserved liver function and a performance status of 0, and early-stage HCC, which includes single HCC or ≤3 nodules, each ≤3 cm in diameter, with preserved liver function and a performance status of 0. The modified Union for International Cancer Control (mUICC) staging system considers the number of tumors, diameter of the largest tumor ≤2 cm, and the absence of vascular or bile duct invasion. Although early-stage HCC is not precisely defined in the mUICC staging system, depending on the number of fulfilled criteria, HCC diagnosed as stage I (all three criteria fulfilled) or stage II (two of three criteria fulfilled, especially in a specific setting without vascular or bile duct invasion) may be regarded as early HCC in the mUICC staging system with respect to the BCLC system and Milan criteria.2,3
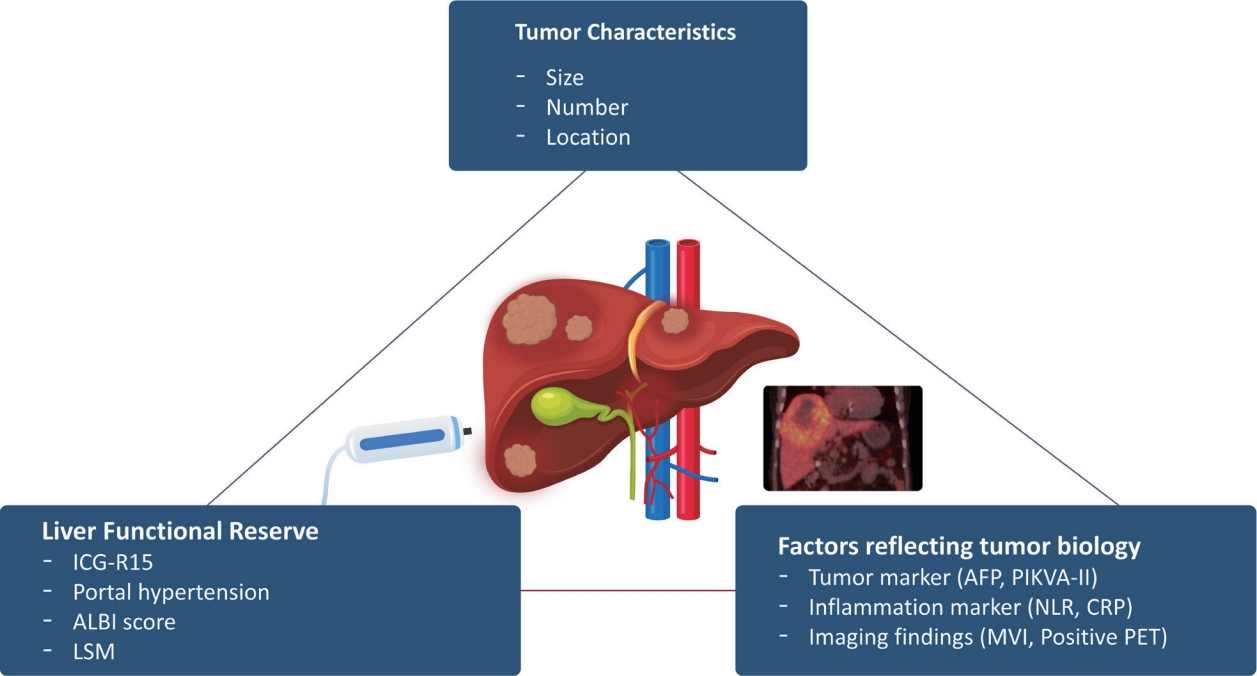
- Treatment methods for early-stage HCC may be categorized into three modalities: liver transplantation (LT), surgical resection (SR), and local ablation.1,4 These three efficient modalities have been explored in various clinical studies and have yielded favorable outcomes in early-stage HCC. However, owing to diverse clinical variables, such as tumor biology, liver functional reserve, patient comorbidities, and the anatomical location of the tumor, routine application of these conventional treatment methods for HCC is not always feasible, resulting in unexpected insufficient anti-tumor responses (Fig. 1).
- In this review, we summarize the challenges in the treatment of early-stage HCC and discuss potential strategies to overcome these hurdles.
INTRODUCTION
- 1. LT
- LT is often recommended for patients who are ineligible for SR when tumor stage falls within the Milan criteria. Theoretically, LT is the most efficient treatment strategy, because it enables removal of not only the tumor tissue but also the cirrhotic liver, thereby preventing de novo carcinogenesis. However, due to shortage of organ availability for patients with early HCC, only living-donor LT (LDLT) is selectively performed in a few centers in South Korea. The Milan criteria, introduced by Mazzaferro et al.,3 include a single tumor ≤5 cm or up to three tumor nodules, each with a maximum diameter of 3 cm, without macrovascular invasion or extrahepatic location. Although the Milan criteria have been associated with a 92% recurrence-free survival (RFS) rate at 4 years, several patients, who may otherwise have favorable prognosis, may be excluded from LT, due to their stringency. Therefore, extended criteria for LT have been proposed with reported favorable outcomes (Table 1). Often, these expanded criteria adapt morphometric variables, such as tumor number and size, to identify specific patients beyond the Milan criteria without increasing the risk of post-transplantation recurrence.5,6 Both the University of California, San Francisco criteria and the Asan criteria have shown positive results in expanding the conventional Milan criteria, although a few conflicting results have been reported, indicating that additional validation is required.
- In addition to morphometric variables, the biology of the tumor is a critical parameter to consider for recurrence after LT, because large or multiple HCCs do not always correlate with poor prognosis. Since pre-transplantation tumor biopsy is not generally applicable due to the risk of tumor cell seeding, and a biopsy may not represent the entire tumor biology due to tumor heterogeneity, predicting post-LT outcomes with noninvasive biomarkers would be valuable.
- Serum alpha-fetoprotein (AFP) and prothrombin induced by vitamin K absence or antagonist-II (PIVKA-II) have been associated with the aggressiveness of tumors.7,8 In particular, AFP has become the most widely used prognostic marker for predicting the aggressiveness of tumors, and some studies have proposed prediction models combined with AFP. Kwon et al.9 and Suh et al.10 emphasized the importance of AFP in assessing the risk and prognosis of HCC using specific thresholds related to survival and recurrence rates. Choi et al.11 also demonstrated that the 5-year disease-free survival (DFS) was 88.6% in patients with serum AFP <100 ng/mL and tumor size <5 cm. Additionally, PIVKA-II, also known as des-γ-carboxy prothrombin, has been suggested as a predictive marker for outcomes after LT, as shown in studies reporting on recurrence and survival rates in different patient groups.12,13 Taketomi investigated the impact of PIVKA-II on HCC recurrence after LDLT and found that the 5-year OS and RFS rates were 82.7% and 87.0%, respectively, in patients with tumors <5 cm in diameter and PIVKA-II <300 mAU/mL.14 Another study from 49 centers including 653 patients found that the 5-year RFS rate was 84.3% in transplant recipients beyond the Milan criteria, serum AFP levels ≤200 ng/mL, and PIVKA-II levels ≤100 mAU/mL.14 Furthermore, Lee et al.7 proposed a model to predict tumor recurrence after LT for HCC beyond Milan criteria (MoRAL score) with serum AFP and PIVKA-II, and demonstrated 5-year recurrence-free and OS rates with MoRAL scores as high as 66.3% and 82.6%, respectively.
- The neutrophil-to-lymphocyte ratio (NLR) is a wellknown marker of active systemic inflammation, and several studies have correlated NLR with recurrence after LT. For instance, Halazun et al.15 found that patients within the Milan criteria and with elevated NLR had significantly poorer DFS relative to those with normal NLR within the Milan criteria (30% vs. 81%; P<0.0001). Similarly, Harimoto et al.16 revealed distinct survival rates based on NLR levels in patients who had undergone LT for HCC.
- Serum C-reactive protein (CRP), a commonly used marker for systemic inflammation, has also been demonstrated as a predictive marker for HCC recurrence after LT. Studies by An et al.17 and Na et al.18 have further highlighted the importance of CRP levels in predicting outcomes, including recurrence and survival, with specific thresholds indicating significantly worse results. The integration of these markers into a newly designed model consisting of NLR and CRP levels has underscored the potential of noninvasive inflammatory markers to predict prognosis in the context of LT for HCC.
- 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) is commonly performed to assess potential extrahepatic metastasis before LT. Research has shown that 18F-FDG PET scanning can help to characterize tumor biology and prognosis after HCC treatment.19,20 Lee et al.21 proposed new criteria using 18F-FDG PET scans and tumor size to select optimal patients and expand the LDLT pool (with negative findings on PET and total tumor size <10 cm). Additional studies have reported that increased 18F-FDG uptake on PET may predict microvascular invasion (MVI) or unfavorable tumor histology, resulting in poor outcomes after LT.22-24
- 2. SR
- Among the curative-intent therapies against HCC, SR has the broadest range of indications relative to other modalities, such as LT and local ablation therapies. SR has shown favorable outcomes for large HCCs, even those >10 cm, when compared to those associated with non-surgical treatments, such as transarterial chemoembolization (TACE), with a fair safety profile.25,26 For example, Hwang et al.25 reported that the 5-year survival rate was 35.5% in 471 patients who had undergone SR for HCC >10 cm, noting that tumor volume did not affect recurrence or survival.
- In cases of multiple HCCs, SR has also shown superior results compared to those associated with alternative treatments such as radiofrequency ablation (RFA) and TACE. Comparative studies have reported better long-term survival with SR for multifocal HCCs than that associated with RFA (hazard ratio [HR], 0.69; P<0.001).27 Moreover, a nationwide survey in Japan including 2,178 patients with multiple HCCs (1,089 patients allocated to each SR and TACE group) demonstrated better 5-year survival rates in the SR group for patients with tumors ≥30 mm in diameter (53.0% vs. 32.7%; P<0.001).28
- SR of HCC is the preferred treatment for patients with a single tumor and preserved liver function, who can survive post-hepatectomy. Therefore, a thorough preoperative assessment of the liver function reserve is crucial to avoid postoperative liver dysfunction (Table 2). The indocyanine green retention rate at 15 minutes (ICG-R15) is commonly used for this purpose, with values under 10% generally recommended, although this may vary depending on the extent of SR.29 Fan et al.30 suggested an ICG-R15 <14% for patients undergoing SR more extensive than right hemi-hepatectomy. Moreover, Schwarz et al.31 analyzed 698 patients who had undergone liver resection for HCC (minor, 44.8%; major, 55.2%) and concluded that indocyanine green <19.5% is the optimal cut-off value for predicting postoperative liver dysfunction. Choi and colleagues demonstrated postoperative hepatic decompensation and poor prognosis in patients with significant portal hypertension (defined by the BCLC criteria as presence of esophageal or gastric varices or splenomegaly [diameter ≥12 cm] with platelet count <100,000/mm3) before SR of HCC.32,33 However, Cucchetti et al.34 demonstrated that the model for end-stage liver disease score and the extent of hepatectomy may server as predictors for postoperative liver failure, regardless of the presence or absence of portal hypertension. The albumin-bilirubin (ALBI) grade has emerged as a reproducible and objective measurement of liver functional reserve in patients with HCC. The ALBI grade defines liver function impairment across three grades and correlates with survival, tumor relapse, and post-hepatectomy liver failure, thus aiding the precise selection of patients suited for SR.35 Lastly, liver stiffness measurement (LSM) by transient elastography is emerging as the most promising noninvasive test to measure liver functional reserve and grade of portal hypertension. Many studies have demonstrated the relationship between LSM and post-hepatectomy liver failure36-38 and Serenari et al.39 recently proposed a nomogram based on LSM to predict postoperative complications in patients with HCC, thus providing a helpful reference for clinicians in decision-making regarding SR.
- Despite promising postsurgical outcomes, the risk of recurrence remains high in specific patient groups. Factors associated with an increased likelihood of recurrence include advanced age, male sex, a high degree of liver dysfunction, and various tumor characteristics such as size, number, grade/differentiation, invasion, presence of satellite lesions, and AFP levels.40,41 To mitigate the risk of suboptimal outcomes following SR, these variables should be considered alongside tumor size and number in determining the suitable HCC treatment modality.
- Recent research has also determined predictive variables for early HCC recurrence after SR, such as sex, albumin-bilirubin grade, and serum AFP.40 Additionally, underlying cirrhosis, more than one nodule and AFP >100 ng/mL have been proposed as preoperative predictors for non-transplantable recurrence after SR.42
- The optimal surgical margins for preventing HCC recurrence after SR were also investigated. Findings suggest that a margin >7 mm is vital for preventing early recurrence, especially in subgroups with specific characteristics such as high AFP levels, non-capsule formation, or MVI.43 Further evidence indicates that wide-margin resection results in better clinical outcomes in patients with solitary HCC ≤5 cm.44
- 3. RFA
- RFA for the treatment of HCC has been studied in various clinical contexts with a focus on tumor size, number, and outcomes.
- Long-term survival rates after RFA against HCC vary depending on tumor size and number (Table 3). Specifically, patients with Child-Pugh class A and HCC ≤2 cm exhibit 65-70% 5-year survival rates, while those with HCC tumor size between 2-5 cm have a 5-year survival rate of 50%.45-47 Comparative studies between RFA and SR have mostly found no significant differences in survival rates.48-50 Even a meta-analysis of eight randomized controlled trials found similar 5-year survival and DFS rates between RFA and liver resection for patients within the Milan criteria.51 However, one recent Korean study found superior DFS with SR, although the OS was similar to that associated with RFA.52 Similarly, other non-randomized studies have found no significant differences in survival rates between liver resection and RFA for the treatment of HCC <3 cm in diameter.53-55
- Efficacy of RFA against HCC may depend on the anatomical location of the tumor. Ideal outcomes occur when the tumor is located distally from the liver capsule, intrahepatic vasculature, or central bile duct, which minimizes complications owing to the heat-sink effect.56,57 Tumors in the subphrenic region may have an increased risk of local recurrence and peritoneal metastasis (up to 9.5%) post-RFA.58,59 When the tumor is close to the large portal or hepatic veins (diameter >3 mm), RFA efficacy may be reduced due to suboptimal heat delivery, with risks of vascular or biliary injury.58,60,61
- MVI is recognized as a predictive factor for poor prognosis following various HCC treatment modalities, including RFA.62,63 Analysis of MVI before treatment is vital for planning and prognostication. Although MVI is typically evaluated based on the microscopic findings of resected tumor specimens, several studies have determined predictive factors for MVI, including non-smooth tumor margins on computed tomography, arterial peritumoral enhancement, hepatobiliary peritumoral hypointensity on magnetic resonance imaging (MRI), increased levels of specific tumor markers (such as AFP and PIVKA-II), and larger tumor size.64-67
PITFALLS FOR INSUFFICIENT TREATMENT OUTCOMES
1) Criteria for transplantation
2) Tumor markers
3) Inflammation markers
4) Positron emission tomography
1) Criteria for SR including clinical variables
2) Liver functional reserve and portal hypertension
3) Factors associated with recurrence after SR
1) Criteria for RFA
2) Technical feasibility of RFA
3) MVI
- 1. LT
- LT is vital for treating HCC; however, it may be challenging to predict and prevent recurrence. Strategies developed to address these challenges are discussed in this section.
- The primary challenge of LT in HCC is the prediction and prevention of post-transplantation HCC recurrence, while ensuring that patients have adequate access to LT. Numerous studies have proposed LT criteria that incorporate not only morphometric variables but also markers reflecting tumor biology (Table 4). By considering an array of variables associated with recurrence and expanding the criteria for LT, accessibility to this procedure may be improved for patients with HCC while maintaining or possibly mitigating recurrence and survival rates.
- For patients unable to undergo LT due to not fulfilling the Milan criteria or characterized by the expected aggressive tumor biology, alternative treatment modalities, such as locoregional therapies, may be advisable. If patients are successfully treated and downstaged within the criteria for LT, they may proceed with the procedure. Additionally, downstaging with locoregional therapy may provide an opportunity to observe the response to therapy and assess tumor biology. A randomized controlled trial investigated 45 patients who were successfully downstaged within the Milan criteria through treatment migration and subsequently underwent LT. The study revealed a 77.5% 5-year OS rate in contrast to a 31.2% rate among patients who did not undergo LT (P=0.035).68
- The objective of HCC downstaging is to meet the acceptable criteria for LT, with the chosen downstaging therapy often dependent on factors such as tumor burden, location, liver function, and center expertise.69,70 TACE is commonly used for downstaging HCC beyond the Milan criteria due to multiple tumor burden and its field effect.71 Both conventional TACE and drug-eluting bead TACE may be employed, although multiple therapy sessions may be necessary for a successful anti-tumor response without residual or recurrent HCC. Yao et al.72 compared the outcomes after tumor downstaging, primarily through TACE followed by LT, in 61 patients who did not meet the Milan criteria. They found successful downstaging in 70.5% of the patients and a 4-year post-transplantation survival rate of 92.1%. A subsequent investigation in 118 patients with HCC who had undergone downstaging within the Milan criteria prior to LT revealed that the 5-year post-transplantation survival rate and the RFS rate were 77.8% and 81.0%, respectively, indicating promising outcomes for patients with HCC who underwent LT after successful downstaging.73
- Transarterial radioembolization (TARE) has been proposed as an effective locoregional therapy for HCC that enables delivery of high-dose radiation to the tumor while minimizing complications such as acute tumor ischemia and liver function deterioration, which are common with TACE. Although data regarding TARE are limited, a study of pooled analysis focusing on the role of TARE in downstaging HCC has shown that both the tumor control rate and response are high, with favorable long-term oncological outcomes after LT.74 TARE for HCC has been endorsed and recommended by various investigators, with Kim et al. reporting higher disease control rates and better survival outcomes in patients without lymph node metastases or distant metastases, specifically with BCLC stage B or C and tumor size ≥5 cm, compared to the same parameters associated with TACE.75 However, the role of TARE in the context of LT and downstaging is still debated due to the absence of clear and unified guidelines.
- Radiotherapy (RT) is a potential treatment modality for downstaging HCC when LT is not an option. Numerous studies have shown that RT is a safe and effective alternative, particularly when other locoregional therapies are unsuitable. A prospective study that compared stereotactic body RT (SBRT) with TACE and high-intensity focused ultrasound as bridge therapies to LT for HCC, revealed that SBRT was not only safe but also had a notably higher tumor control rate and low risk of waitlist dropout.76-78 The role of RT in bridge therapy to LT for HCC with portal vein tumor thrombosis (PVTT) has also been explored. It has been suggested that LT following RT could be the treatment of choice for HCC compounded with PVTT in certain patients.79,80 Moreover, the efficacy of neoadjuvant RT prior to LT for HCC that is not amenable to locoregional treatment has been examined. Although a complete pathological response was noted in 47.8% of patients undergoing RT, the 5-year survival rate was not significantly different from that of patients who did not undergo RT (68.7% vs. 61.7%; P=0.829).81
- 2. SR
- Eligibility for SR depends on various factors such as tumor burden, liver dysfunction, portal hypertension, and planned resection scope, while considering future liver remnants.82 SR has traditionally been limited to patients with underlying cirrhosis, significant portal hypertension, or inadequate future liver remnants. However, the recent emergence of minimally invasive surgical (MIS) approaches, along with advances in intraoperative and perioperative management, has increased the number of patients eligible for SR. MIS approaches, including laparoscopic and robot-assisted liver resection, are typically used for limited minor resections in anatomically favorable locations, with major liver resection generally restricted to high-volume centers. Meta-analyses of comparative studies have shown that MIS approaches resulted in reduced surgical blood loss, shorter hospital stay, and decreased 30-day morbidity, compared to the same parameters associated with open resection, while RFS and OS rates appear comparable.83,84
- Transarterial therapy is often recommended for intermediate-stage HCC; however, favorable responses have been reported for early-stage HCC. Retrospective studies with patients within the Milan criteria who underwent SR, RFA, or TACE have found that the TACE group experienced a shorter time to tumor progression, with no significant difference in the 5-year survival rate between groups.53,85 Thus, TACE may serve as an alternative curative option for early-stage HCC when surgical approaches or RFA are not feasible. Additionally, TARE may be an alternative with an efficacy comparable to that of TACE in a neoadjuvant setting, particularly for patients with lobar portal vein thrombosis. TARE provides benefits over TACE, such as reduced toxicity and contralateral remnant liver hypertrophy, without the need for portal vein embolization.86-88 Thus, TARE is becoming an increasingly preferred method for downstaging patients before surgical intervention.
- RT has been proposed as a downstaging tool for HCC when SR is not possible, especially in locally advanced HCC such as PVTT.89,90 Chong et al.91 compared the disease-specific survival rate in patients receiving SR after localized concurrent chemoradiotherapy with those receiving SR only and found improved survival in the concurrent chemoradiotherapy group (62 vs. 15 months; P=0.006). Multivariate analysis further determined that the radiological response may influence survival. In addition, a randomized controlled study by Wei et al.92 assessed neoadjuvant RT in patients with resectable HCC and PVTT and revealed a significantly higher 2-year survival rate in the neoadjuvant RT group than in the surgery-alone group (27.4% vs. 9.4%; P<0.001), indicating enhanced postoperative outcomes with neoadjuvant RT.
- Although SR increases the possibility of long-term survival in patients with HCC, the risk of postoperative recurrence remains significant, emphasizing the need for neoadjuvant or adjuvant therapies. In the treatment of various malignant tumors, the use of neoadjuvant or adjuvant therapies with the goal of downstaging the disease to enable resection or improve postoperative outcomes is common. However, the contribution of neoadjuvant and adjuvant therapies in the treatment of HCC is yet to be determined. Preoperative TACE as neoadjuvant therapy has generated conflicting results. A meta-analysis including 32 studies concluded that there was no notable difference in DFS or OS between patients who received preoperative TACE and those who did not. Nevertheless, patients who demonstrated complete response to TACE exhibited significantly improved DFS and OS. Furthermore, a prospective study including 67 patients with early-stage HCC, who were not candidates for LT or SR but underwent TACE, showed a complete anti-tumor response rate of 67.2% after a month and a 3-year survival rate of 80.5%.93
- The STORM study, which investigated the efficacy of sorafenib (a tyrosine kinase inhibitor) as adjuvant therapy after SR for HCC in 900 patients across 28 countries, found no difference in RFS between patients treated with postoperative sorafenib and those who did not. Sorafenib was associated with a poor safety profile (including four drug-related deaths). Although currently no randomized trials have evaluated the efficacy of lenvatinib (another first-line systemic tyrosine kinase inhibitor agent for HCC) as an adjuvant therapy in the postoperative setting, small retrospective studies have indicated improved survival outcomes in selected high-risk patient cohorts presenting with MVI.94,95
- Finally, the IMBrave050 trial was the first study where adjuvant therapy showed a tangible benefit in terms of patient outcomes. In this open-label randomized clinical trial, administration of adjuvant atezolizumab and bevacizumab for 12 months significantly improved RFS in high-risk patients.96 Furthermore, phase I and II studies employing a combination of immune checkpoint inhibitors (including nivolumab + cabozantinib, and nivolumab ± ipilimumab), yielded major pathologic responses in 30-40% of patients. Despite these encouraging results, further exploration through phase III studies and large-scale real-world data is essential to comprehensively understand the roles of neoadjuvant and adjuvant therapies with immune checkpoint inhibitors in the management of HCC.
- 3. RFA
- RFA generally yields favorable outcomes for the treatment of single tumors ≤2 cm in diameter, but for tumors within the Milan criteria, RFA has been associated with a higher recurrence rate than that associated with SR, despite significantly lower complication rates. If patients are suspected to have high-risk MVI, SR of HCC has shown superior 5-year recurrence and OS rate compared to that associated with RFA for early-stage HCC.97 Consequently, RFA should be considered for patients more likely to have a positive treatment outcome or those who cannot undergo invasive treatments such as SR. In such cases, combination treatment with TACE may be considered.98,99 Chen et al.100 compared the post-recurrence survival and OS rates in patients with resectable HCC who underwent RFA or TACE, and found no significant difference in MVI association between the groups.
- Technical feasibility of RFA can be enhanced with additional methods. When the tumor is adjacent to other organs, RFA can be performed safely and effectively using artificial ascites to create a barrier between the tumor and neighboring structures.101 When a tumor is not adequately visualized through conventional B-mode ultrasonography, contrastenhanced ultrasonography or navigation fusion imaging may enhance detection and response rates.102,103 In a Korean prospective study involving 216 patients with HCCs <5 cm in diameter, 30/76 HCCs (39.5%) that were not visible on B-mode ultrasonography were successfully located using fusion imaging. Furthermore, all 60 HCCs that were unsuitable for RFA on B-mode ultrasonography were rendered feasible for RFA when fusion imaging was applied.102 Contrast-enhanced ultrasonography has also been shown to improve the detection rate of small HCCs that are difficult to be detected on Bmode ultrasonography, with higher detection rates associated with contrast-enhanced ultrasonography under fusion imaging compared to those associated with contrast-enhanced ultrasonography alone.103 Regarding perivascular HCC, Lee et al.104 demonstrated better DFS associated with microwave ablation relative to RFA, although the 2-year survival rate was not significantly different between the two. Moreover, recent advancements in RFA technology have led to the development of no-touch RFA, which involves the placement of multiple electrodes around the periphery of the tumor. This technique has been shown to result in lower local recurrence rate than that associated with conventional RFA, which directly punctures the tumor.105,106 However, further research is required to ensure that this approach also confers a survival advantage.107
- SBRT may be an effective alternative when RFA is not feasible. A retrospective study by Kim et al.108 that compared the treatment efficacy between RFA and SBRT found that SBRT was associated with a significantly lower risk of local recurrence than that associated with RFA (HR, 0.36; P<0.001) and provided superior local control for small tumors (≤3 cm), regardless of location, and in large tumors in the subphrenic region. Another retrospective study by Shin et al.109 found no significant difference between SBRT and RFA in terms of OS and local tumor control rate. Although more prospective randomized trials are required, SBRT may be the preferred treatment option when RFA is not an option. A randomized phase III trial investigating the treatment efficacy of RFA and proton beam radiotherapy (PBT) against recurrent/residual HCC demonstrated that the 2-year local progression-free survival rate was 92.8% in the PBT group and 83.2% in the RFA group, proving the non-inferiority of PBT to RFA. Furthermore, another network meta-analysis comparing the efficacy of treatment modalities such as PBT and RFA in early-stage HCC showed no significant difference between modalities, suggesting that PBT may be an alternative treatment option when RFA is not feasible.
- As alternatives to RFA, application of microwave ablation (MWA) and cryoablation against early-stage HCC has been increasing. Since microwaves can readily penetrate biologic materials continuously applying high temperatures (>150℃), MWA can improve ablation efficacy by increasing thermal conduction into the surrounding tissue.110 Two randomized controlled studies comparing the RFS, and OS between MWA and RFA in patients with HCC (tumor number ≤3 and tumor size ≤ 4-5 cm) demonstrated no significant difference,111,112 and meta-analyses also showed no difference between the two groups regarding OS and major complication rates.113,114 However, microwave energy is inherently more difficult to distribute than radiofrequency energy is, limiting its application in clinical practice.115
- Cryoablation has the merit of feasibility for treatment monitoring, because ice balls can be easily identified with ultrasound, non-enhanced CT, or MRI. A multicenter randomized controlled trial comparing cryoablation and RFA in HCC by Wang et al.116 exhibited no significant difference in 1-, 3-, and 5-year OS, RFS, and major complication rates. Although some studies have reported lower complication rate in cryoablation relative to RFA in HCC located near the bile duct or vascular structure, the size of the cryoablated zone is known to correlate with probe diameter.117,118 Hence, in circumstances when a large ablation zone is required, multiple or large-diameter probes with prolonged treatment time are required.115
STRATEGIES TO ADDRESS SUBOPTIMAL OUTCOMES
1) Incorporation of criteria with tumor biologic markers
2) Treatment migration and downstaging
3) Downstaging of HCC: TACE
4) Downstaging of HCC: transarterial radioembolization
5) Downstaging of HCC: radiotherapy
1) Minimally invasive surgical approach
2) Treatment migration: transarterial therapy
3) Treatment migration: RT
4) Neoadjuvant and adjuvant therapy
1) MVI
2) Overcoming technical feasibility issues
3) SBRT
4) Microwave ablation and cryoablation
- Although HCC generally has a poor prognosis, favorable outcomes are possible in patients with early-stage HCC via highly effective treatment modalities, such as LT, SR, and local ablation. However, achieving optimal results with these treatments presents numerous challenges. Management of HCC requires a holistic approach, considering not only the number and size of tumors, but also the underlying tumor biology, residual liver function, and various risk factors that may influence recurrence and survival outcomes. These approaches are only feasible with multidisciplinary approaches with experts in diverse fields of HCC gathering novel opinions on the most optimal treatment strategy. By developing precise criteria for each treatment modality and personalizing approaches based on liver function and tumor-related variables, therapeutic outcomes for patients with early-stage HCC may be enhanced.
SUMMARY AND PERSPECTIVES
-
Conflict of Interest
The authors have no conflicts of interest to disclose.
-
Ethics Statement
This review article is fully based on articles which have already been published and did not involve additional patient participants. Therefore, IRB approval is not necessary.
-
Funding Statement
None.
-
Data Availability
Not applicable.
-
Author Contribution
Conceptualization: JHY
Supervision: SKC
Writing–original draft: JHY
Writing–review & editing: SKC
Article information
Acknowledgments
| Marker | Study, criterion name | Value for cut-off | HCC characteristic | 5-year RFS (%) | 5-year OS (%) |
|---|---|---|---|---|---|
| AFP | DuBay et al.,119 Toronto | <400 | hCC confined to the liver and no poor histologic differentiation | 66 | 70 |
| Toso et al.,120 Toso | ≤400 | Total tumor volume ≤115 cm3 | 68.0* | 74.6* | |
| Wan et al.121 | ≤400 | Tumor ≤10 cm, no vascular and extrahepatic invasions | 74.4 | 73.7 | |
| Duvoux et al.,122 French | ≤100 | Nodule diameters ≤3 cm, between 3-6 cm, or ≥6 cm | 66.6 | 69.9 | |
| PIVKA-II | Kaido et al.,123 Kyoto | ≤400 | Up to 10 hCCs with a diameter ≤5 cm | - | 82.0 |
| Ito et al.124 | ≤400 | Tumor size ≤10 cm | - | 86.7 | |
| Soejima et al.125 | ≤300 | Tumor size ≤5 cm | 93.8† | - | |
| AFP/PIVKA-II | Lee et al.,7 MoRAL | AFP and PIVKA-II derived score | Beyond Milan criteria | 66.3 | 86.0 |
| Todo et al.14 | AFP ≤200, PIVKA-II ≤100 | Milan criteria | 96.4 | - | |
| Shindoh et al.126 | AFP ≤250, PIVKA-II ≤450 | Tokyo criteria | 96.8 | - | |
| NLR/CRP | Na et al.18 | NLR < 6.0 and CRP < 1.0 | None | 96.8 | 84.0 |
- 1. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, GarciaCriado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 2022;76:681−693.ArticlePubMedPMC
- 2. Ueno S, Tanabe G, Nuruki K, Hamanoue M, Komorizono Y, Oketani M, et al. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res 2002;24:395−403.ArticlePubMed
- 3. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693−699.ArticlePubMed
- 4. Korean Liver Cancer Association (KLCA), National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol 2022;28:583−705.ArticlePubMedPMCPDF
- 5. Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl 2008;14:935−945.ArticlePubMed
- 6. Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant 2007;7:2587−2596.ArticlePubMed
- 7. Lee JH, Cho Y, Kim HY, Cho EJ, Lee DH, Yu SJ, et al. Serum tumor markers provide refined prognostication in selecting liver transplantation candidate for hepatocellular carcinoma patients beyond the Milan criteria. Ann Surg 2016;263:842−850.ArticlePubMed
- 8. Notarpaolo A, Layese R, Magistri P, Gambato M, Colledan M, Magini G, et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J Hepatol 2017;66:552−559.ArticlePubMed
- 9. Kwon CH, Kim DJ, Han YS, Park JB, Choi GS, Kim SJ, et al. HCC in living donor liver transplantation: can we expand the Milan criteria? Dig Dis 2007;25:313−319.ArticlePubMedPDF
- 10. Suh KS, Cho EH, Lee HW, Shin WY, Yi NJ, Lee KU. Liver transplantation for hepatocellular carcinoma in patients who do not meet the Milan criteria. Dig Dis 2007;25:329−333.ArticlePubMedPDF
- 11. Choi HJ, Kim DG, Na GH, Han JH, Hong TH, You YK. Clinical outcome in patients with hepatocellular carcinoma after living-donor liver transplantation. World J Gastroenterol 2013;19:4737−4744.ArticlePubMedPMC
- 12. Carr BI, Kanke F, Wise M, Satomura S. Clinical evaluation of lens culinaris agglutinin-reactive alpha-fetoprotein and des-gammacarboxy prothrombin in histologically proven hepatocellular carcinoma in the United States. Dig Dis Sci 2007;52:776−782.ArticlePubMedPDF
- 13. Shirabe K, Itoh S, Yoshizumi T, Soejima Y, Taketomi A, Aishima S, et al. The predictors of microvascular invasion in candidates for liver transplantation with hepatocellular carcinoma-with special reference to the serum levels of des-gamma-carboxy prothrombin. J Surg Oncol 2007;95:235−240.ArticlePubMed
- 14. Todo S, Furukawa H, Tada M; Japanese Liver Transplantation Study Group. Extending indication: role of living donor liver transplantation for hepatocellular carcinoma. Liver Transpl 2007;13 Suppl 2:S48−S54.Article
- 15. Halazun KJ, Hardy MA, Rana AA, Woodland DC 4th, Luyten EJ, Mahadev S, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg 2009;250:141−151.ArticlePubMed
- 16. Harimoto N, Shirabe K, Nakagawara H, Toshima T, Yamashita Y, Ikegami T, et al. Prognostic factors affecting survival at recurrence of hepatocellular carcinoma after living-donor liver transplantation: with special reference to neutrophil/lymphocyte ratio. Transplantation 2013;96:1008−1012.PubMed
- 17. An HJ, Jang JW, Bae SH, Choi JY, Yoon SK, Lee MA, et al. Serum C-reactive protein is a useful biomarker for predicting outcomes after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl 2012;18:1406−1414.ArticlePubMedPDF
- 18. Na GH, Kim DG, Han JH, Kim EY, Lee SH, Hong TH, et al. Inflammatory markers as selection criteria of hepatocellular carcinoma in living-donor liver transplantation. World J Gastroenterol 2014;20:6594−6601.ArticlePubMedPMC
- 19. Kornberg A, Schernhammer M, Friess H. 18F-FDG-PET for assessing biological viability and prognosis in liver transplant patients with hepatocellular carcinoma. J Clin Transl Hepatol 2017;5:224−234.ArticlePubMedPMC
- 20. Yaprak O, Acar S, Ertugrul G, Dayangac M. Role of pre-transplant 18F-FDG PET/CT in predicting hepatocellular carcinoma recurrence after liver transplantation. World J Gastrointest Oncol 2018;10:336−343.ArticlePubMedPMC
- 21. Lee SD, Lee B, Kim SH, Joo J, Kim SK, Kim YK, et al. Proposal of new expanded selection criteria using total tumor size and (18) F-fluorodeoxyglucose - positron emission tomography/computed tomography for living donor liver transplantation in patients with hepatocellular carcinoma: The National Cancer Center Korea criteria. World J Transplant 2016;6:411−422.ArticlePubMedPMC
- 22. Ling LL, Hsu CC, Yong CC, Elsarawy AM, Chan YC, Wang CC, et al. FDG-PET predicted unfavorable tumor histology in living donor liver transplant recipients: a retrospective cohort study. Int J Surg 2019;69:124−131.ArticlePubMed
- 23. Kornberg A, Freesmeyer M, Bärthel E, Jandt K, Katenkamp K, Steenbeck J, et al. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant 2009;9:592−600.ArticlePubMed
- 24. Yang SH, Suh KS, Lee HW, Cho EH, Cho JY, Cho YB, et al. The role of (18)F-FDG-PET imaging for the selection of liver transplantation candidates among hepatocellular carcinoma patients. Liver Transpl 2006;12:1655−1660.ArticlePubMed
- 25. Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY, et al. Longterm outcome after resection of huge hepatocellular carcinoma ≥ 10 cm: single-institution experience with 471 patients. World J Surg 2015;39:2519−2528.ArticlePubMedPDF
- 26. Wei CY, Chen PC, Chau GY, Lee RC, Chen PH, Huo TI, et al. Comparison of prognosis between surgical resection and transarterial chemoembolization for patients with solitary huge hepatocellular carcinoma. Ann Transl Med 2020;8:238. ArticlePubMedPMC
- 27. Yue YY, Zhou WL. Hepatic resection is associated with improved long-term survival compared to radio-frequency ablation in patients with multifocal hepatocellular carcinoma. Front Oncol 2020;10:110. ArticlePubMedPMC
- 28. Fukami Y, Kaneoka Y, Maeda A, Kumada T, Tanaka J, Akita T, et al. Liver resection for multiple hepatocellular carcinomas: a Japanese nationwide survey. Ann Surg 2020;272:145−154.PubMed
- 29. Makuuchi M, Sano K. The surgical approach to HCC: our progress and results in Japan. Liver Transpl 2004;10 Suppl 1:S46−S52.Article
- 30. Fan ST, Lai EC, Lo CM, Ng IO, Wong J. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg 1995;130:198−203.ArticlePubMed
- 31. Schwarz C, Plass I, Fitschek F, Punzengruber A, Mittlböck M, Kampf S, et al. The value of indocyanine green clearance assessment to predict postoperative liver dysfunction in patients undergoing liver resection. Sci Rep 2019;9:8421. ArticlePubMedPMCPDF
- 32. Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329−338.ArticlePubMed
- 33. Choi GH, Park JY, Hwang HK, Kim DH, Kang CM, Choi JS, et al. Predictive factors for long-term survival in patients with clinically significant portal hypertension following resection of hepatocellular carcinoma. Liver Int 2011;31:485−493.ArticlePubMed
- 34. Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, La Barba G, et al. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl 2006;12:966−971.ArticlePubMed
- 35. Demirtas CO, D’Alessio A, Rimassa L, Sharma R, Pinato DJ. ALBI grade: evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP Rep 2021;3:100347. ArticlePubMedPMC
- 36. Cescon M, Colecchia A, Cucchetti A, Peri E, Montrone L, Ercolani G, et al. Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann Surg 2012;256:706−712. discussion 712-713.ArticlePubMed
- 37. Kim SU, Kim BK, Han KH. Clinical application of liver stiffness measurement using transient elastography: a surgical perspective. Digestion 2013;88:258−265.ArticlePubMedPDF
- 38. Rajakannu M, Cherqui D, Ciacio O, Golse N, Pittau G, Allard MA, et al. Liver stiffness measurement by transient elastography predicts late posthepatectomy outcomes in patients undergoing resection for hepatocellular carcinoma. Surgery 2017;162:766−774.ArticlePubMed
- 39. Serenari M, Han KH, Ravaioli F, Kim SU, Cucchetti A, Han DH, et al. A nomogram based on liver stiffness predicts postoperative complications in patients with hepatocellular carcinoma. J Hepatol 2020;73:855−862.ArticlePubMed
- 40. Chan AWH, Zhong J, Berhane S, Toyoda H, Cucchetti A, Shi K, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol 2018;69:1284−1293.ArticlePubMed
- 41. Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 2015;261:947−955.PubMed
- 42. Gelli M, Sebagh M, Porcher R, Romanelli E, Vibert E, Sa Cunha A, et al. Liver resection for early hepatocellular carcinoma: preoperative predictors of non transplantable recurrence and implications for treatment allocation. Ann Surg 2020;272:820−826.PubMed
- 43. Nitta H, Allard MA, Sebagh M, Golse N, Ciacio O, Pittau G, et al. Ideal surgical margin to prevent early recurrence after hepatic resection for hepatocellular carcinoma. World J Surg 2021;45:1159−1167.ArticlePubMedPDF
- 44. Wang H, Yu H, Qian YW, Cao ZY, Wu MC, Cong WM. Impact of surgical margin on the prognosis of early hepatocellular carcinoma (≤5 cm): a propensity score matching analysis. Front Med (Lausanne) 2020;7:139. ArticlePubMedPMC
- 45. Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol 2013;58:89−97.ArticlePubMed
- 46. Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol 2012;107:569−577. quiz 578.ArticlePubMedPMCPDF
- 47. Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology 2008;47:82−89.ArticlePubMed
- 48. Feng K, Yan J, Li X, Xia F, Ma K, Wang S, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012;57:794−802.ArticlePubMed
- 49. Ng KKC, Chok KSH, Chan ACY, Cheung TT, Wong TCL, Fung JYY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg 2017;104:1775−1784.ArticlePubMedPDF
- 50. Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321−328.ArticlePubMedPMC
- 51. Wang Q, Tang M, Zhang S. Comparison of radiofrequency ablation and surgical resection for hepatocellular carcinoma conforming to the Milan criteria: a meta-analysis. ANZ J Surg 2021;91:E432−E438.ArticlePubMedPDF
- 52. Lee HW, Lee JM, Yoon JH, Kim YJ, Park JW, Park SJ, et al. A prospective randomized study comparing radiofrequency ablation and hepatic resection for hepatocellular carcinoma. Ann Surg Treat Res 2018;94:74−82.ArticlePubMedPMCPDF
- 53. Yang HJ, Lee JH, Lee DH, Yu SJ, Kim YJ, Yoon JH, et al. Small single-nodule hepatocellular carcinoma: comparison of transarterial chemoembolization, radiofrequency ablation, and hepatic resection by using inverse probability weighting. Radiology 2014;271:909−918.ArticlePubMed
- 54. Kang TW, Kim JM, Rhim H, Lee MW, Kim YS, Lim HK, et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection--Propensity score analyses of long-term outcomes. Radiology 2015;275:908−919.ArticlePubMed
- 55. Kim GA, Shim JH, Kim MJ, Kim SY, Won HJ, Shin YM, et al. Radiofrequency ablation as an alternative to hepatic resection for single small hepatocellular carcinomas. Br J Surg 2016;103:126−135.ArticlePubMedPDF
- 56. Lee DH, Kim JW, Lee JM, Kim JM, Lee MW, Rhim H, et al. Laparoscopic liver resection versus percutaneous radiofrequency ablation for small single nodular hepatocellular carcinoma: comparison of treatment outcomes. Liver Cancer 2021;10:25−37.ArticlePubMedPMCPDF
- 57. de Baère T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol 2003;181:695−700.ArticlePubMed
- 58. Lee MW, Kang D, Lim HK, Cho J, Sinn DH, Kang TW, et al. Updated 10-year outcomes of percutaneous radiofrequency ablation as first-line therapy for single hepatocellular carcinoma <3 cm: emphasis on association of local tumor progression and overall survival. Eur Radiol 2020;30:2391−2400.ArticlePubMedPDF
- 59. Song KD, Lim HK, Rhim H, Lee MW, Kang TW, Paik YH, et al. Hepatic resection vs percutaneous radiofrequency ablation of hepatocellular carcinoma abutting right diaphragm. World J Gastrointest Oncol 2019;11:227−237.ArticlePubMedPMC
- 60. Lee S, Kang TW, Cha DI, Song KD, Lee MW, Rhim H, et al. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: propensity score analyses of long-term outcomes. J Hepatol 2018;69:70−78.ArticlePubMed
- 61. Kang TW, Lim HK, Lee MW, Kim YS, Rhim H, Lee WJ, et al. Aggressive intrasegmental recurrence of hepatocellular carcinoma after radiofrequency ablation: risk factors and clinical significance. Radiology 2015;276:274−285.ArticlePubMed
- 62. Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol 2008;15:1375−1382.ArticlePubMedPDF
- 63. Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg 2011;254:108−113.ArticlePubMed
- 64. Renzulli M, Brocchi S, Cucchetti A, Mazzotti F, Mosconi C, Sportoletti C, et al. Can current preoperative imaging be used to detect microvascular invasion of hepatocellular carcinoma? Radiology 2016;279:432−442.ArticlePubMed
- 65. Imai K, Yamashita YI, Yusa T, Nakao Y, Itoyama R, Nakagawa S, et al. Microvascular invasion in small-sized hepatocellular carcinoma: significance for outcomes following hepatectomy and radiofrequency ablation. Anticancer Res 2018;38:1053−1060.PubMed
- 66. Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol 2015;62:848−854.ArticlePubMed
- 67. Lee S, Kang TW, Song KD, Lee MW, Rhim H, Lim HK, et al. Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann Surg 2021;273:564−571.ArticlePubMed
- 68. Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol 2020;21:947−956.ArticlePubMed
- 69. Yao FY, Fidelman N. Reassessing the boundaries of liver transplantation for hepatocellular carcinoma: where do we stand with tumor down-staging? Hepatology 2016;63:1014−1025.ArticlePubMedPDF
- 70. Rudnick SR, Russo MW. Liver transplantation beyond or downstaging within the Milan criteria for hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol 2018;12:265−275.ArticlePubMed
- 71. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int 2015;35:2155−2166.PubMedPMC
- 72. Yao FY, Kerlan RK Jr, Hirose R, Davern TJ 3rd, Bass NM, Feng S, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology 2008;48:819−827.PubMed
- 73. Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology 2015;61:1968−1977.PubMed
- 74. Lopez-Lopez V, Miura K, Kuemmerli C, Capel A, Eshmuminov D, Ferreras D, et al. Selecting the appropriate downstaging and bridging therapies for hepatocellular carcinoma: what is the role of transarterial radioembolization? a pooled analysis. Cancers (Basel) 2023;15:2122. ArticlePubMedPMC
- 75. Kim MA, Jang H, Choi NR, Nam JY, Lee YB, Cho EJ, et al. Yttrium90 radioembolization is associated with better clinical outcomes in patients with hepatocellular carcinoma compared with conventional chemoembolization: a propensity score-matched study. J Hepatocell Carcinoma 2021;8:1565−1577.ArticlePubMedPMCPDF
- 76. Sandroussi C, Dawson LA, Lee M, Guindi M, Fischer S, Ghanekar A, et al. Radiotherapy as a bridge to liver transplantation for hepatocellular carcinoma. Transpl Int 2010;23:299−306.ArticlePubMed
- 77. Mohkam K, Golse N, Bonal M, Ledochowski S, Rode A, Selmaji IE, et al. Conformal radiotherapy as a bridge to liver transplantation for hepatocellular carcinoma: is it safe? Future Oncol 2016;12:1577−1586.ArticlePubMed
- 78. Wong TC, Lee VH, Law AL, Pang HH, Lam KO, Lau V, et al. Prospective study of stereotactic body radiation therapy for hepatocellular carcinoma on waitlist for liver transplant. Hepatology 2021;74:2580−2594.ArticlePubMedPMCPDF
- 79. Choi JY, Yu JI, Park HC, Kwon CHD, Kim JM, Joh JW, et al. The possibility of radiotherapy as downstaging to living donor liver transplantation for hepatocellular carcinoma with portal vein tumor thrombus. Liver Transpl 2017;23:545−551.ArticlePubMedPDF
- 80. Han DH, Joo DJ, Kim MS, Choi GH, Choi JS, Park YN, et al. Living donor liver transplantation for advanced hepatocellular carcinoma with portal vein tumor thrombosis after concurrent chemoradiation therapy. Yonsei Med J 2016;57:1276−1281.ArticlePubMedPMCPDF
- 81. Mourad M, Mabrut JY, Chellakhi M, Lesurtel M, Prevost C, Ducerf C, et al. Neoadjuvant conformal radiotherapy before liver transplantation for hepatocellular carcinoma: a propensity score matched analysis of postoperative morbidity and oncological results. Future Oncol 2019;15:2517−2530.ArticlePubMed
- 82. Citterio D, Facciorusso A, Sposito C, Rota R, Bhoori S, Mazzaferro V. Hierarchic interaction of factors associated with liver decompensation after resection for hepatocellular carcinoma. JAMA Surg 2016;151:846−853.ArticlePubMed
- 83. Witowski J, Rubinkiewicz M, Mizera M, Wysocki M, Gajewska N, Sitkowski M, et al. Meta-analysis of short- and long-term outcomes after pure laparoscopic versus open liver surgery in hepatocellular carcinoma patients. Surg Endosc 2019;33:1491−1507.ArticlePubMedPMCPDF
- 84. Ciria R, Gomez-Luque I, Ocaña S, Cipriani F, Halls M, Briceño J, et al. A systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for hepatocellular carcinoma: updated results from the European guidelines meeting on laparoscopic liver surgery, Southampton, UK, 2017. Ann Surg Oncol 2019;26:252−263.ArticlePubMedPDF
- 85. Oh JH, Sinn DH, Choi GS, Kim JM, Joh JW, Kang TW, et al. Comparison of outcome between liver resection, radiofrequency ablation, and transarterial therapy for multiple small hepatocellular carcinoma within the Milan criteria. Ann Surg Treat Res 2020;99:238−246.ArticlePubMedPMCPDF
- 86. Vouche M, Lewandowski RJ, Atassi R, Memon K, Gates VL, Ryu RK, et al. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol 2013;59:1029−1036.ArticlePubMedPMC
- 87. Braat AJAT, Huijbregts JE, Molenaar IQ, Rinkes IHMB, van den Bosch MAAJ, Lam MGEH. Hepatic radioembolization as a bridge to liver surgery. Front Oncol 2014;4:199. ArticlePubMedPMC
- 88. Gaba RC, Lewandowski RJ, Kulik LM, Riaz A, Ibrahim SM, Mulcahy MF, et al. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol 2009;16:1587−1596.ArticlePubMedPDF
- 89. Lee HS, Choi GH, Choi JS, Kim KS, Han KH, Seong J, et al. Surgical resection after down-staging of locally advanced hepatocellular carcinoma by localized concurrent chemoradiotherapy. Ann Surg Oncol 2014;21:3646−3653.ArticlePubMedPDF
- 90. Hamaoka M, Kobayashi T, Kuroda S, Iwako H, Okimoto S, Kimura T, et al. Hepatectomy after down-staging of hepatocellular carcinoma with portal vein tumor thrombus using chemoradiotherapy: a retrospective cohort study. Int J Surg 2017;44:223−228.ArticlePubMed
- 91. Chong JU, Choi GH, Han DH, Kim KS, Seong J, Han KH, et al. Downstaging with localized concurrent chemoradiotherapy can identify optimal surgical candidates in hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol 2018;25:3308−3315.ArticlePubMedPDF
- 92. Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol 2019;37:2141−2151.ArticlePubMedPMC
- 93. Bargellini I, Sacco R, Bozzi E, Bertini M, Ginanni B, Romano A, et al. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: a prospective cohort study. Eur J Radiol 2012;81:1173−1178.ArticlePubMed
- 94. Bai S, Hu L, Liu J, Sun M, Sun Y, Xue F. Prognostic nomograms combined adjuvant lenvatinib for hepatitis B virus-related hepatocellular carcinoma with microvascular invasion after radical resection. Front Oncol 2022;12:919824. ArticlePubMedPMC
- 95. Dai MG, Liu SY, Lu WF, Liang L, Ye B. Survival benefits from adjuvant lenvatinib for patients with hepatocellular carcinoma and microvascular invasion after curative hepatectomy. Clin Med Insights Oncol 2023;17:11795549231180351. ArticlePubMedPMCPDF
- 96. Hack SP, Spahn J, Chen M, Cheng AL, Kaseb A, Kudo M, et al. IMbrave 050: a phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol 2020;16:975−989.ArticlePubMed
- 97. Bai S, Yang P, Xie Z, Li J, Lei Z, Xia Y, et al. Preoperative estimated risk of microvascular invasion is associated with prognostic differences following liver resection versus radiofrequency ablation for early hepatitis B virus-related hepatocellular carcinoma. Ann Surg Oncol 2021;28:8174−8185.ArticlePubMedPDF
- 98. Wang Y, Deng T, Zeng L, Chen W. Efficacy and safety of radiofrequency ablation and transcatheter arterial chemoembolization for treatment of hepatocellular carcinoma: a meta-analysis. Hepatol Res 2016;46:58−71.ArticlePubMed
- 99. Yan S, Xu D, Sun B. Combination of radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: a meta-analysis. Dig Dis Sci 2013;58:2107−2113.ArticlePubMedPDF
- 100. Chen SL, Xiao H, Xie ZL, Shen JX, Chen ZB, Wang YQ, et al. The presence of microvascular invasion guides treatment strategy in recurrent HBV-related HCC. Eur Radiol 2020;30:3473−3485.ArticlePubMedPDF
- 101. Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol 2009;19:2630−2640.ArticlePubMedPDF
- 102. Ahn SJ, Lee JM, Lee DH, Lee SM, Yoon JH, Kim YJ, et al. Real-time US-CT/MR fusion imaging for percutaneous radiofrequency ablation of hepatocellular carcinoma. J Hepatol 2017;66:347−354.ArticlePubMed
- 103. Calandri M, Mauri G, Yevich S, Gazzera C, Basile D, Gatti M, et al. Fusion imaging and virtual navigation to guide percutaneous thermal ablation of hepatocellular carcinoma: a review of the literature. Cardiovasc Intervent Radiol 2019;42:639−647.ArticlePubMedPDF
- 104. Lee SK, Chung DJ, Cho SH. A real-world comparative study of microwave and radiofrequency ablation in treatment-naïve and recurrent hepatocellular carcinoma. J Clin Med 2022;11:302. ArticlePubMedPMC
- 105. Park SJ, Cho EJ, Lee JH, Yu SJ, Kim YJ, Yoon JH, et al. Switching monopolar no-touch radiofrequency ablation using octopus electrodes for small hepatocellular carcinoma: a randomized clinical trial. Liver Cancer 2021;10:72−81.ArticlePubMedPDF
- 106. Suh YS, Choi JW, Yoon JH, Lee DH, Kim YJ, Lee JH, et al. No-touch vs. conventional radiofrequency ablation using twin internally cooled wet electrodes for small hepatocellular carcinomas: a randomized prospective comparative study. Korean J Radiol 2021;22:1974−1984.ArticlePubMedPMCPDF
- 107. Lee DH, Lee MW, Kim PN, Lee YJ, Park HS, Lee JM. Outcome of no-touch radiofrequency ablation for small hepatocellular carcinoma: a multicenter clinical trial. Radiology 2021;301:229−236.ArticlePubMed
- 108. Kim N, Cheng J, Jung I, Liang J, Shih YL, Huang WY, et al. Stereotactic body radiation therapy vs. radiofrequency ablation in Asian patients with hepatocellular carcinoma. J Hepatol 2020;73:121−129.ArticlePubMed
- 109. Shin HS, Lee SH, Jun BG, Kim HS, Kang SH, Park JY, et al. Stereotactic body radiotherapy versus radiofrequency ablation as initial treatment of small hepatocellular carcinoma. Eur J Gastroenterol Hepatol 2022;34:1187−1194.ArticlePubMed
- 110. Yang D, Converse MC, Mahvi DM, Webster JG. Measurement and analysis of tissue temperature during microwave liver ablation. IEEE Trans Biomed Eng 2007;54:150−155.ArticlePubMed
- 111. Violi NV, Duran R, Guiu B, Cercueil JP, Aubé C, Digklia A, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol 2018;3:317−325.ArticlePubMed
- 112. Chong CCN, Lee KF, Cheung SYS, Chu CCM, Fong AKW, Wong J, et al. Prospective double-blinded randomized controlled trial of microwave versus radiofrequency ablation for hepatocellular carcinoma (McRFA trial). HPB (Oxford) 2020;22:1121−1127.ArticlePubMed
- 113. Gupta P, Maralakunte M, Kumar-M P, Chandel K, Chaluvashetty SB, Bhujade H, et al. Overall survival and local recurrence following RFA, MWA, and cryoablation of very early and early HCC: a systematic review and Bayesian network meta-analysis. Eur Radiol 2021;31:5400−5408.ArticlePubMedPDF
- 114. Tan W, Deng Q, Lin S, Wang Y, Xu G. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma : a systematic review and meta-analysis. Int J Hyperthermia 2019;36:264−272.ArticlePubMedPDF
- 115. Ahmed M, Brace CL, Lee FT Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology 2011;258:351−369.ArticlePubMedPMC
- 116. Wang C, Wang H, Yang W, Hu K, Xie H, Hu KQ, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology 2015;61:1579−1590.ArticlePubMed
- 117. Kim R, Kang TW, Cha DI, Song KD, Lee MW, Rhim H, et al. Percutaneous cryoablation for perivascular hepatocellular carcinoma: therapeutic efficacy and vascular complications. Eur Radiol 2019;29:654−662.ArticlePubMedPDF
- 118. Ko SE, Lee MW, Rhim H, Kang TW, Song KD, Cha DI, et al. Comparison of procedure-related complications between percutaneous cryoablation and radiofrequency ablation for treating periductal hepatocellular carcinoma. Int J Hyperthermia 2020;37:1354−1361.ArticlePubMed
- 119. DuBay D, Sandroussi C, Sandhu L, Cleary S, Guba M, Cattral MS, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg 2011;253:166−172.ArticlePubMed
- 120. Toso C, Meeberg G, Hernandez-Alejandro R, Dufour JF, Marotta P, Majno P, et al. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: a prospective validation. Hepatology 2015;62:158−165.ArticlePubMedPDF
- 121. Wan P, Xia Q, Zhang JJ, Li QG, Xu N, Zhang M, et al. Liver transplantation for hepatocellular carcinoma exceeding the Milan criteria: a single-center experience. J Cancer Res Clin Oncol 2014;140:341−348.ArticlePubMedPDF
- 122. Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986−994.e3. quiz e14-e15.ArticlePubMed
- 123. Kaido T, Ogawa K, Mori A, Fujimoto Y, Ito T, Tomiyama K, et al. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery 2013;154:1053−1060.ArticlePubMed
- 124. Ito T, Takada Y, Ueda M, Haga H, Maetani Y, Oike F, et al. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transpl 2007;13:1637−1644.ArticlePubMed
- 125. Soejima Y, Taketomi A, Yoshizumi T, Uchiyama H, Aishima S, Terashi T, et al. Extended indication for living donor liver transplantation in patients with hepatocellular carcinoma. Transplantation 2007;83:893−899.ArticlePubMed
- 126. Shindoh J, Sugawara Y, Nagata R, Kaneko J, Tamura S, Aoki T, et al. Evaluation methods for pretransplant oncologic markers and their prognostic impacts in patient undergoing living donor liver transplantation for hepatocellular carcinoma. Transpl Int 2014;27:391−398.ArticlePubMed
References
Figure & Data
References
Citations

- Diosgenin potentiates the anticancer effect of doxorubicin and volasertib via regulating polo-like kinase 1 and triggering apoptosis in hepatocellular carcinoma cells
Eman H. Yousef, Mohamed E. El-Mesery, Maha R. Habeeb, Laila A. Eissa
Naunyn-Schmiedeberg's Archives of Pharmacology.2024; 397(7): 4883. CrossRef - Comparison of Surgical Resection and Radiofrequency Ablation in Elderly Patients with Hepatocellular Carcinoma
Jun Il Kim, Jayoun Lee, Gi Hong Choi, Min Woo Lee, Dong Ah Park, Jeong-Ju Yoo
Digestive Diseases and Sciences.2024; 69(3): 1055. CrossRef - Radiofrequency for hepatocellular carcinoma larger than 3 cm: potential for applications in daily practice
Ji Hoon Kim, Pil Soo Sung
Journal of Liver Cancer.2024; 24(1): 1. CrossRef - Hepatocellular carcinoma outcomes and potential implications for surveillance in elderly patients
Aryoung Kim, Goeun Park, Myung Ji Goh, Byeong Geun Song, Wonseok Kang, Geum-Youn Gwak, Yong-Han Paik, Moon Seok Choi, Joon Hyeok Lee, Dong Hyun Sinn
Scientific Reports.2024;[Epub] CrossRef - Trends in alcohol use and alcoholic liver disease in South Korea: a nationwide cohort study
Jeong-Ju Yoo, Dong Hyeon Lee, Young Chang, Hoongil Jo, Young Youn Cho, Sangheun Lee, Log Young Kim, Jae Young Jang
BMC Public Health.2024;[Epub] CrossRef - Efficacy of Transarterial Chemoembolization (TACE) for Early-Stage Hepatocellular Carcinoma
Moonhyung Lee, Hyun Phil Shin
Medicina.2023; 59(12): 2174. CrossRef
 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation
- Download Citation
- Close
- Related articles
-
- Intermediate-stage hepatocellular carcinoma: refining substaging or shifting paradigm?
- Radioembolization for hepatocellular carcinoma: what clinicians need to know
- Liver transplantation for hepatocellular carcinoma with portal vein tumor thrombosis
- A Case of Lymphocyte-Rich Hepatocellular Carcinoma in a Patient Who Was Treated for Colon Cancer

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION


 Follow JLC on Twitter
Follow JLC on Twitter