Most viewed
- Page Path
- HOME > Articles and issues > Most viewed
Guideline
- 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma
- J Liver Cancer. 2023;23(1):1-120. Published online December 9, 2022
- DOI: https://doi.org/10.17998/jlc.2022.11.07
- 9,693 Views
- 333 Downloads
- 34 Citations
-
 Abstract
Abstract
 PDF
PDF - Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the fourth most common cancer among men in South Korea, where the prevalence of chronic hepatitis B infection is high in middle and old age. The current practice guidelines will provide useful and sensible advice for the clinical management of patients with HCC. A total of 49 experts in the fields of hepatology, oncology, surgery, radiology, and radiation oncology from the Korean Liver Cancer Association-National Cancer Center Korea Practice Guideline Revision Committee revised the 2018 Korean guidelines and developed new recommendations that integrate the most up-to-date research findings and expert opinions. These guidelines provide useful information and direction for all clinicians, trainees, and researchers in the diagnosis and treatment of HCC.
-
Citations
Citations to this article as recorded by- Contrast-enhanced US for HCC: Finally out from the waiting list?
Richard G. Barr, Luigi Bolondi
Hepatology.2024; 79(2): 267. CrossRef - The Epidemiology of Hepatitis B Virus Infection in Korea: 15-Year Analysis
Log Young Kim, Jeong-Ju Yoo, Young Chang, Hoongil Jo, Young Youn Cho, Sangheun Lee, Dong Hyeon Lee, Jae Young Jang
Journal of Korean Medical Science.2024;[Epub] CrossRef - Potential role of Fibrosis‐4 score in hepatocellular carcinoma screening: The Kangbuk Samsung Health Study
Sujeong Shin, Won Sohn, Yoosoo Chang, Yoosun Cho, Min‐Jung Kwon, Sarah H. Wild, Christopher D. Byrne, Seungho Ryu
Hepatology Research.2024; 54(6): 551. CrossRef - Research Progress of lncRNA-ATB/miR-141-3p/GP73 Ax-is-Mediated EMT Promoting TACE Refractoriness
棋 耿
Advances in Clinical Medicine.2024; 14(01): 903. CrossRef - Response to atezolizumab plus bevacizumab specific for lung and lymph node metastases affects survival of patients with HCC
Jiwon Yang, Jonggi Choi, Won‐Mook Choi, Kang Mo Kim, Han Chu Lee, Ju Hyun Shim
Liver International.2024; 44(4): 907. CrossRef - Comparison of Surgical Resection and Radiofrequency Ablation in Elderly Patients with Hepatocellular Carcinoma
Jun Il Kim, Jayoun Lee, Gi Hong Choi, Min Woo Lee, Dong Ah Park, Jeong-Ju Yoo
Digestive Diseases and Sciences.2024; 69(3): 1055. CrossRef - Management Consensus Guidelines for Hepatocellular Carcinoma: 2023 Update on Surveillance, Diagnosis, Systemic Treatment, and Posttreatment Monitoring by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan
Wei Teng, Hung-Wei Wang, Shi-Ming Lin
Liver Cancer.2024; : 1. CrossRef - Complications of immunotherapy in advanced hepatocellular carcinoma
Young-Gi Song, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
Journal of Liver Cancer.2024; 24(1): 9. CrossRef - Liver resection in selective hepatocellular carcinoma with Vp3 or Vp4 portal vein tumor thrombosis improves prognosis
Manuel Lim, Jongman Kim, Jinsoo Rhu, Gyu-Seong Choi, Jae-Won Joh
Journal of Liver Cancer.2024; 24(1): 102. CrossRef - Comparison of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma with portal vein tumor thrombosis
Jeayeon Park, Yun Bin Lee, Yunmi Ko, Youngsu Park, Hyunjae Shin, Moon Haeng Hur, Min Kyung Park, Dae-Won Lee, Eun Ju Cho, Kyung-Hun Lee, Jeong-Hoon Lee, Su Jong Yu, Tae-Yong Kim, Yoon Jun Kim, Tae-You Kim, Jung-Hwan Yoon
Journal of Liver Cancer.2024; 24(1): 81. CrossRef - Programmed Death 1 and Cytotoxic T-Lymphocyte-Associated Protein 4 Gene Expression in Peripheral Blood Mononuclear Cells Can Serve as Prognostic Biomarkers for Hepatocellular Carcinoma
Ji Ah Lee, Hei-Gwon Choi, Hyuk Soo Eun, Jiyoon Bu, Tae Min Jang, Jeongdong Lee, Chae Yeon Son, Min Seok Kim, Woo Sun Rou, Seok Hyun Kim, Byung Seok Lee, Ha Neul Kim, Tae Hee Lee, Hong Jae Jeon
Cancers.2024; 16(8): 1493. CrossRef - Risk of Hepatocellular Carcinoma by Steatotic Liver Disease and Its Newly Proposed Subclassification
Byeong Geun Song, Aryoung Kim, Myung Ji Goh, Wonseok Kang, Geum-Youn Gwak, Yong-Han Paik, Moon Seok Choi, Joon Hyeok Lee, Dong Hyun Sinn
Liver Cancer.2024; : 1. CrossRef - Efficacy and Safety of Surgical Resection in Elderly Patients with Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
Jin-Soo Lee, Dong Ah Park, Seungeun Ryoo, Jungeun Park, Gi Hong Choi, Jeong-Ju Yoo
Gut and Liver.2024; 18(4): 695. CrossRef - Consistent efficacy of hepatic artery infusion chemotherapy irrespective of PD‑L1 positivity in unresectable hepatocellular carcinoma
Ji Kim, Young Kim, Hee-Chul Nam, Chang-Wook Kim, Jae-Sung Yoo, Ji Han, Jeong Jang, Jong Choi, Seung Yoon, Ho Jong Chun, Jung Oh, Suho Kim, Sung Lee, Pil Sung
Oncology Letters.2024;[Epub] CrossRef - Risk of Bleeding in Hepatocellular Carcinoma Patients Treated with Atezolizumab/Bevacizumab: A Systematic Review and Meta-Analysis
Young-Gi Song, Kyeong-Min Yeom, Eun Ae Jung, Sang Gyune Kim, Young Seok Kim, Jeong-Ju Yoo
Liver Cancer.2024; : 1. CrossRef - Trends in alcohol use and alcoholic liver disease in South Korea: a nationwide cohort study
Jeong-Ju Yoo, Dong Hyeon Lee, Young Chang, Hoongil Jo, Young Youn Cho, Sangheun Lee, Log Young Kim, Jae Young Jang
BMC Public Health.2024;[Epub] CrossRef - Fatal intratumoral hemorrhage in a patient with hepatocellular carcinoma following successful treatment with atezolizumab/bevacizumab: A case report
Kyeong-Hoon Park, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
World Journal of Clinical Cases.2024; 12(22): 5177. CrossRef - Diagnostic performance of the 2022 KLCA-NCC criteria for hepatocellular carcinoma on magnetic resonance imaging with extracellular contrast and hepatobiliary agents: comparison with the 2018 KLCA-NCC criteria
Ja Kyung Yoon, Sunyoung Lee, Jeong Ah Hwang, Ji Eun Lee, Seung-seob Kim, Myeong-Jin Kim
Journal of Liver Cancer.2023; 23(1): 157. CrossRef - Radiologic features of hepatocellular carcinoma related to prognosis
Shin Hye Hwang, Hyungjin Rhee
Journal of Liver Cancer.2023; 23(1): 143. CrossRef - Current status of ultrasonography in national cancer surveillance program for hepatocellular carcinoma in South Korea: a large-scale multicenter study
Sun Hong Yoo, Soon Sun Kim, Sang Gyune Kim, Jung Hyun Kwon, Han-Ah Lee, Yeon Seok Seo, Young Kul Jung, Hyung Joon Yim, Do Seon Song, Seong Hee Kang, Moon Young Kim, Young-Hwan Ahn, Jieun Han, Young Seok Kim, Young Chang, Soung Won Jeong, Jae Young Jang, J
Journal of Liver Cancer.2023; 23(1): 189. CrossRef - Smoking Increases the Risk of Hepatocellular Carcinoma and Cardiovascular Disease in Patients with Metabolic-Associated Fatty Liver Disease
Jeong-Ju Yoo, Man Young Park, Eun Ju Cho, Su Jong Yu, Sang Gyune Kim, Yoon Jun Kim, Young Seok Kim, Jung-Hwan Yoon
Journal of Clinical Medicine.2023; 12(9): 3336. CrossRef - Transarterial Chemoembolization for Hepatocellular Carcinoma: 2023 Expert Consensus-Based Practical Recommendations of the Korean Liver Cancer Association
Yuri Cho, Jin Woo Choi, Hoon Kwon, Kun Yung Kim, Byung Chan Lee, Hee Ho Chu, Dong Hyeon Lee, Han Ah Lee, Gyoung Min Kim, Jung Suk Oh, Dongho Hyun, In Joon Lee, Hyunchul Rhim
Korean Journal of Radiology.2023; 24(7): 606. CrossRef - Transarterial chemoembolization for hepatocellular carcinoma: 2023 expert consensus-based practical recommendations of the Korean Liver Cancer Association
Yuri Cho, Jin Woo Choi, Hoon Kwon, Kun Yung Kim, Byung Chan Lee, Hee Ho Chu, Dong Hyeon Lee, Han Ah Lee, Gyoung Min Kim, Jung Suk Oh, Dongho Hyun, In Joon Lee, Hyunchul Rhim
Journal of Liver Cancer.2023; 23(2): 241. CrossRef - Transarterial chemoembolization for hepatocellular carcinoma: 2023 Expert consensus-based practical recommendations of the Korean Liver Cancer Association
Yuri Cho, Jin Woo Choi, Hoon Kwon, Kun Yung Kim, Byung Chan Lee, Hee Ho Chu, Dong Hyeon Lee, Han Ah Lee, Gyoung Min Kim, Jung Suk Oh, Dongho Hyun, In Joon Lee, Hyunchul Rhim
Clinical and Molecular Hepatology.2023; 29(3): 521. CrossRef - Classification of microvascular invasion of hepatocellular carcinoma: correlation with prognosis and magnetic resonance imaging
Yoon Jung Hwang, Jae Seok Bae, Youngeun Lee, Bo Yun Hur, Dong Ho Lee, Haeryoung Kim
Clinical and Molecular Hepatology.2023; 29(3): 733. CrossRef - A nationwide study on the current treatment status and natural prognosis of hepatocellular carcinoma in elderly
Jeong-Ju Yoo, Jayoun Lee, Gi Hong Choi, Min Woo Lee, Dong Ah Park
Scientific Reports.2023;[Epub] CrossRef - Diagnosis of hepatocellular carcinoma using Sonazoid: a comprehensive review
Woo Kyoung Jeong
Journal of Liver Cancer.2023; 23(2): 272. CrossRef - The Current Evidence of Intensity-Modulated Radiotherapy for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
Won Il Jang, Sunmi Jo, Ji Eun Moon, Sun Hyun Bae, Hee Chul Park
Cancers.2023; 15(20): 4914. CrossRef - Clinical outcome of surgical resection for multifocal T2-T3 hepatocellular carcinoma up to 3 nodules: a comparative analysis with a single nodule
Sehyeon Yu, Hye-Sung Jo, Young-Dong Yu, Yoo jin Choi, Dong-Sik Kim
Journal of Liver Cancer.2023; 23(2): 377. CrossRef - Sonazoid contrast-enhanced ultrasonography for the diagnosis of hepatocellular carcinoma: strengths and shortcomings
Sung Won Lee, Min Kyu Kang, Xiang Zhang
Journal of Liver Cancer.2023; 23(2): 238. CrossRef - Sequential regorafenib or nivolumab therapy in recurrent hepatocellular carcinoma with sorafenib failure in liver transplant patients does not improve prognosis
Jieun Kwon, Jongman Kim, Jinsoo Rhu, Gyu-Seong Choi, Jae-Won Joh
Annals of Liver Transplantation.2023; 3(2): 104. CrossRef - Concurrent nivolumab and external beam radiation therapy for hepatocellular carcinoma with macrovascular invasion: A phase 2 study
Bo Hyun Kim, Hee Chul Park, Tae Hyun Kim, Young-Hwan Koh, Jung Yong Hong, Yuri Cho, Dong Hyun Sinn, Boram Park, Joong-Won Park
JHEP Reports.2023; : 100991. CrossRef - Regular Alpha-Fetoprotein Tests Boost Curative Treatment and Survival for Hepatocellular Carcinoma Patients in an Endemic Area
Joo Hyun Oh, Jonghyun Lee, Eileen L. Yoon, Soung Won Jeong, Soon Sun Kim, Young Eun Chon, Sang Bong Ahn, Dae Won Jun
Cancers.2023; 16(1): 150. CrossRef - Adjuvant and neoadjuvant therapies for hepatocellular carcinoma
Arndt Vogel, Robert C. Grant, Tim Meyer, Gonzalo Sapisochin, Grainne M. O’Kane, Anna Saborowski
Hepatology.2023;[Epub] CrossRef
- Contrast-enhanced US for HCC: Finally out from the waiting list?

Review Articles
- Radioembolization for hepatocellular carcinoma: what clinicians need to know
- Jin Woo Choi, Hyo-Cheol Kim
- J Liver Cancer. 2022;22(1):4-13. Published online February 23, 2022
- DOI: https://doi.org/10.17998/jlc.2022.01.16

- 9,476 Views
- 491 Downloads
- 9 Citations
-
 Abstract
Abstract
 PDF
PDF - Transarterial radioembolization (TARE) with yttrium 90 (90Y) has been used in the management of hepatocellular carcinoma (HCC) for more than 10 years in Korea. There are two types of 90Y radioactive microspheres available, namely, glass and resin microspheres, with comparable clinical outcomes. In general, TARE outperforms transarterial chemoembolization regarding post-embolization syndrome, time to progression, tumor downsizing for liver transplantation, and hospitalization stay. Although TARE is commonly recommended for patients with unresectable large HCCs, it can be an alternative to or performed in combination with ablation, surgical resection, and systemic treatment. This review aimed to address 90Y radioactive microspheres, patient selection, clinical outcomes, simulation tests, radioembolization procedures, follow-up imaging, and complications.
-
Citations
Citations to this article as recorded by- Unlocking Precision in Radioembolization: Navigating the Future of Holmium-166 Radioembolization Mapping and Lung Shunt Study by Implementing Scout Dosimetry
Peiman Habibollahi, Armeen Mahvash, Nima Kokabi, Nariman Nezami
CardioVascular and Interventional Radiology.2024; 47(4): 451. CrossRef - Feasibility of Liver Transplantation after 90Y Radioembolization: Lessons from a Radiation Protection Incident
Marine Soret, Jacques-Antoine Maisonobe, Philippe Maksud, Stéphane Payen, Manon Allaire, Eric Savier, Charles Roux, Charlotte Lussey-Lepoutre, Aurélie Kas
Health Physics.2024; 127(3): 373. CrossRef - Liver-Directed Locoregional Therapies for Neuroendocrine Liver Metastases: Recent Advances and Management
Cody R. Criss, Mina S. Makary
Current Oncology.2024; 31(4): 2076. CrossRef - Selective internal radiation therapy segmentectomy: A new minimally invasive curative option for primary liver malignancies?
Riccardo Inchingolo, Francesco Cortese, Antonio Rosario Pisani, Fabrizio Acquafredda, Roberto Calbi, Riccardo Memeo, Fotis Anagnostopoulos, Stavros Spiliopoulos
World Journal of Gastroenterology.2024; 30(18): 2379. CrossRef - Transarterial chemoembolization as an alternative to radioembolization is associated with earlier tumor recurrence than in radioembolization-eligible patients
Sung Won Chung, Heejin Cho, Hyunjae Shin, Jeayeon Park, Ju Yeon Kim, Ji Hoon Hong, Moon Haeng Hur, Min Kyung Park, Yun Bin Lee, Su Jong Yu, Myungsu Lee, Yoon Jun Kim, Jin Chul Paeng, Jung-Hwan Yoon, Jin Wook Chung, Jeong-Hoon Lee, Hyo-Cheol Kim
Frontiers in Oncology.2023;[Epub] CrossRef - The evolution of immune checkpoint inhibitor combinations in advanced hepatocellular carcinoma – A systematic review
Brandon M. Meyers, Jennifer J. Knox, David M. Liu, Deanna McLeod, Ravi Ramjeesingh, Vincent C. Tam, Howard J. Lim
Cancer Treatment Reviews.2023; 118: 102584. CrossRef - Recent Advances in Image-Guided Locoregional Therapies for Primary Liver Tumors
Cody R. Criss, Mina S. Makary
Biology.2023; 12(7): 999. CrossRef - Multidisciplinary consensus recommendations for management of hepatocellular carcinoma in Middle East and North Africa region
Imam Waked, Sherif Alsammany, Sayed Hammad Tirmazy, Kakil Rasul, Jafar Bani‐Issa, Wael Abdel‐Razek, Ashraf Omar, Amr Shafik, Salem Eid, Amr Abdelaal, Ahmed Hosni, Gamal Esmat
Liver International.2023; 43(10): 2062. CrossRef - Impact of Low Skeletal Muscle Mass on Long-Term Outcomes in Hepatocellular Carcinoma Treated with Trans-Arterial Radioembolization: A Retrospective Multi-Center Study
Heechul Nam, Hyun Yang, Ho Soo Chun, Han Ah Lee, Joon Yeul Nam, Jeong Won Jang, Yeon Seok Seo, Do Young Kim, Yoon Jun Kim, Si Hyun Bae
Cancers.2023; 15(21): 5195. CrossRef
- Unlocking Precision in Radioembolization: Navigating the Future of Holmium-166 Radioembolization Mapping and Lung Shunt Study by Implementing Scout Dosimetry

- A clinical and pathological update on hepatocellular carcinoma
- Salvatore Lorenzo Renne, Luca Di Tommaso
- J Liver Cancer. 2022;22(1):14-22. Published online March 31, 2022
- DOI: https://doi.org/10.17998/jlc.2022.03.18

- 8,996 Views
- 536 Downloads
- 12 Citations
-
 Abstract
Abstract
 PDF
PDF - It is estimated that more than 1 million individuals will be affected annually by hepatocellular carcinoma (HCC) by 2025. HCC can be broadly grouped into two major molecular subgroups, each of which is characterized by specific morphological and phenotypic features that mirror the genetic background. The use of these tissue biomarkers in the daily practice of pathologists promises to better allocate patients with HCC with adequate treatments. In turn, this will likely boost the attitude of clinicians toward obtaining a pre-treatment biopsy.
-
Citations
Citations to this article as recorded by- Radiofrequency Ablation versus Surgical Resection in Elderly Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
Jeong-Ju Yoo, Sujin Koo, Gi Hong Choi, Min Woo Lee, Seungeun Ryoo, Jungeun Park, Dong Ah Park
Current Oncology.2024; 31(1): 324. CrossRef - Comparison of Surgical Resection and Radiofrequency Ablation in Elderly Patients with Hepatocellular Carcinoma
Jun Il Kim, Jayoun Lee, Gi Hong Choi, Min Woo Lee, Dong Ah Park, Jeong-Ju Yoo
Digestive Diseases and Sciences.2024; 69(3): 1055. CrossRef - Enhancing liver cirrhosis varices and CSPH risk prediction with spleen stiffness measurement using 100-Hz probe
Jeong-Ju Yoo, Sun Ah Maeng, Young Chang, Sae Hwan Lee, Soung Won Jeong, Jae Young Jang, Gab Jin Cheon, Young Seok Kim, Hong Soo Kim, Sang Gyune Kim
Scientific Reports.2024;[Epub] CrossRef - Identification of gallbladder cancer by direct near-infrared measurement of raw bile combined with two-trace two-dimensional correlation analysis
Eunjin Jang, Woosuk Sohng, Dongho Choi, Hoeil Chung
The Analyst.2023; 148(2): 374. CrossRef - Measurement of Heavy Metal and Antioxidant-Oxidant Levels in Tissues Obtained From Three Different Localizations of Explant Hepatectomy of Patients With Hepatocellular Carcinoma
Cemalettin Koc, Sami Akbulut, Kemal Baris Sarici, Muhammed Mehdi Uremis, Ufuk Gunay Dogan, Zeynep Kucukakcali, Ibrahim Umar Garzali, Ertugrul Karabulut, Yusuf Turkoz, Sezai Yilmaz
Transplantation Proceedings.2023; 55(5): 1262. CrossRef - Adding MRI as a Surveillance Test for Hepatocellular Carcinoma in Patients with Liver Cirrhosis Can Improve Prognosis
Su Jong Yu, Jeong-Ju Yoo, Dong Ho Lee, Su Jin Kim, Eun Ju Cho, Se Hyung Kim, Jeong-Hoon Lee, Yoon Jun Kim, Jeong Min Lee, Jae Young Lee, Jung-Hwan Yoon
Biomedicines.2023; 11(2): 382. CrossRef - Development of a sorafenib-loaded solid self-nanoemulsifying drug delivery system: Formulation optimization and characterization of enhanced properties
Chaemin Lim, Dayoon Lee, Mikyung Kim, Subin Lee, Yuseon Shin, Jacob D. Ramsey, Han-Gon Choi, Eun Seong Lee, Yu Seok Youn, Kyung Taek Oh
Journal of Drug Delivery Science and Technology.2023; 82: 104374. CrossRef - Validation of MELD 3.0 scoring system in East Asian patients with cirrhosis awaiting liver transplantation
Jeong-Ju Yoo, Jong-In Chang, Ji Eun Moon, Dong Hyun Sinn, Sang Gyune Kim, Young Seok Kim
Liver Transplantation.2023; 29(10): 1029. CrossRef - Higher Number of Tumor-Infiltrating PD-L1+ Cells Is Related to Better Response to Multikinase Inhibitors in Hepatocellular Carcinoma
Ji Won Han, Ji Hoon Kim, Dong Hyun Kim, Jeong Won Jang, Si Hyun Bae, Jong Young Choi, Seung Kew Yoon, Jaegyoon Ahn, Hyun Yang, Pil Soo Sung
Diagnostics.2023; 13(8): 1453. CrossRef - Risk of dyslipidemia in chronic hepatitis B patients taking tenofovir alafenamide: a systematic review and meta-analysis
Eui Gwon Hwang, Eun-Ae Jung, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
Hepatology International.2023; 17(4): 860. CrossRef - Recent application of artificial intelligence on histopathologic image-based prediction of gene mutation in solid cancers
Mohammad Rizwan Alam, Kyung Jin Seo, Jamshid Abdul-Ghafar, Kwangil Yim, Sung Hak Lee, Hyun-Jong Jang, Chan Kwon Jung, Yosep Chong
Briefings in Bioinformatics.2023;[Epub] CrossRef - Role of pelitinib in the regulation of migration and invasion of hepatocellular carcinoma cells via inhibition of Twist1
Sewoong Lee, Eunjeong Kang, Unju Lee, Sayeon Cho
BMC Cancer.2023;[Epub] CrossRef
- Radiofrequency Ablation versus Surgical Resection in Elderly Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis

- Recent updates on the classification of hepatoblastoma according to the International Pediatric Liver Tumors Consensus
- Se Un Jeong, Hyo Jeong Kang
- J Liver Cancer. 2022;22(1):23-29. Published online March 17, 2022
- DOI: https://doi.org/10.17998/jlc.2022.02.24
- 6,592 Views
- 522 Downloads
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF - Hepatoblastoma is the most common pediatric liver malignancy and usually occurs within the first 3 years of life. In recent years, the overall incidence of hepatoblastoma has exhibited the greatest increase among all pediatric malignancies worldwide. The diagnosis of hepatoblastoma may be challenging due to the lack of a current consensus classification system. The International Pediatric Liver Tumors Consensus introduced guidelines and a consensus classification for the diagnosis of hepatoblastoma as either epithelial or mixed epithelial and mesenchymal and in the updated 5th edition of the World Health Organization Classification of Digestive System Tumors.
-
Citations
Citations to this article as recorded by- Tumor‐associated macrophages: The key player in hepatoblastoma microenvironment and the promising therapeutic target
Ahmad Adawy, Yoshihiro Komohara, Taizo Hibi
Microbiology and Immunology.2024;[Epub] CrossRef - Adult hepatoblastoma: making the challenging distinction from hepatocellular carcinoma
Allison Kaye L. Pagarigan, Paulo Giovanni L. Mendoza
Journal of Liver Cancer.2023; 23(1): 219. CrossRef - Advances in Histological and Molecular Classification of Hepatocellular Carcinoma
Joon Hyuk Choi, Swan N. Thung
Biomedicines.2023; 11(9): 2582. CrossRef
- Tumor‐associated macrophages: The key player in hepatoblastoma microenvironment and the promising therapeutic target

- Combination of interventional oncology local therapies and immunotherapy for the treatment of hepatocellular carcinoma
- Dong-Hyun Kim
- J Liver Cancer. 2022;22(2):93-102. Published online April 22, 2022
- DOI: https://doi.org/10.17998/jlc.2022.03.28
- 6,088 Views
- 181 Downloads
- 7 Citations
-
 Abstract
Abstract
 PDF
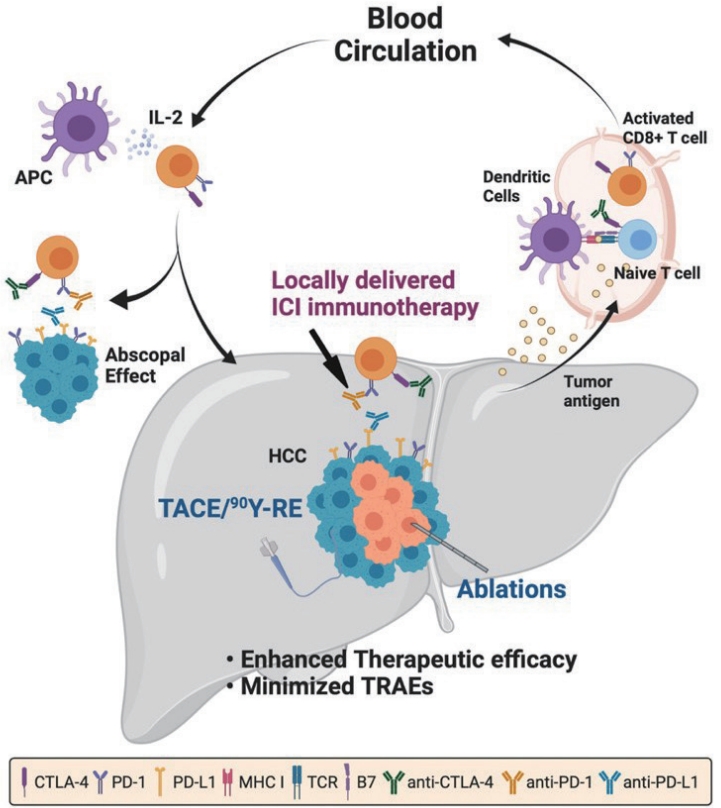
PDF - Interventional oncology (IO) local therapies of hepatocellular carcinoma (HCC) can activate anti-cancer immunity and it is potentially leading to an anti-cancer immunity throughout the body. For the development of an effective HCC treatment regime, great emphasis has been dedicated to different IO local therapy mediated immune modulation and possible combinations with immune checkpoint inhibitor immunotherapy. In this review paper, we summarize the status of combination of IO local therapy and immunotherapy, as well as the prospective role of therapeutic carriers and locally administered immunotherapy in advanced HCC.
-
Citations
Citations to this article as recorded by- Reduced-Dose or Discontinuation of Bevacizumab Might Be Considered after Variceal Bleeding in Patients with Hepatocellular Carcinoma Receiving Atezolizumab/Bevacizumab: Case Reports
Kyeong-Min Yeom, Young-Gi Song, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
Medicina.2024; 60(1): 157. CrossRef - CT-guided high dose rate brachytherapy can induce multiple systemic proteins of proliferation and angiogenesis predicting outcome in HCC
Lukas Salvermoser, Shraga Nahum Goldberg, Marianna Alunni-Fabbroni, Philipp Maximilian Kazmierczak, Moritz Nikolaus Gröper, Jan Niklas Schäfer, Elif Öcal, Tanja Burkard, Stefanie Corradini, Najib Ben Khaled, Agnese Petrera, Moritz Wildgruber, Jens Ricke,
Translational Oncology.2024; 43: 101919. CrossRef - Complications of immunotherapy in advanced hepatocellular carcinoma
Young-Gi Song, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
Journal of Liver Cancer.2024; 24(1): 9. CrossRef - Syngeneic N1-S1 Orthotopic Hepatocellular Carcinoma in Sprague Dawley Rat for the Development of Interventional Oncology-Based Immunotherapy: Survival Assay and Tumor Immune Microenvironment
Bongseo Choi, Jason Pe, Bo Yu, Dong-Hyun Kim
Cancers.2023; 15(3): 913. CrossRef - Preclinical Development and Validation of Translational Temperature Sensitive Iodized Oil Emulsion Mediated Transcatheter Arterial Chemo‐Immuno‐Embolization for the Treatment of Hepatocellular Carcinoma
Heegon Kim, Bongseo Choi, Samdeep K. Mouli, Hyunjun Choi, Kathleen R. Harris, Laura M. Kulik, Robert J. Lewandowski, Dong‐Hyun Kim
Advanced Healthcare Materials.2023;[Epub] CrossRef - The Current Evidence of Intensity-Modulated Radiotherapy for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
Won Il Jang, Sunmi Jo, Ji Eun Moon, Sun Hyun Bae, Hee Chul Park
Cancers.2023; 15(20): 4914. CrossRef - Inducing the Abscopal Effect in Liver Cancer Treatment: The Impact of Microwave Ablation Power Levels and PD-1 Antibody Therapy
Changli Liao, Guiyuan Zhang, Ruotong Huang, Linyuan Zeng, Bin Chen, Haitao Dai, Keyu Tang, Run Lin, Yonghui Huang
Pharmaceuticals.2023; 16(12): 1672. CrossRef
- Reduced-Dose or Discontinuation of Bevacizumab Might Be Considered after Variceal Bleeding in Patients with Hepatocellular Carcinoma Receiving Atezolizumab/Bevacizumab: Case Reports

- Non-alcoholic fatty liver disease-related hepatocellular carcinoma
- Darine Daher, Karim Seif El Dahan, Amit G. Singal
- J Liver Cancer. 2023;23(1):127-142. Published online February 9, 2023
- DOI: https://doi.org/10.17998/jlc.2022.12.30
- 4,769 Views
- 205 Downloads
- 10 Citations
-
 Abstract
Abstract
 PDF
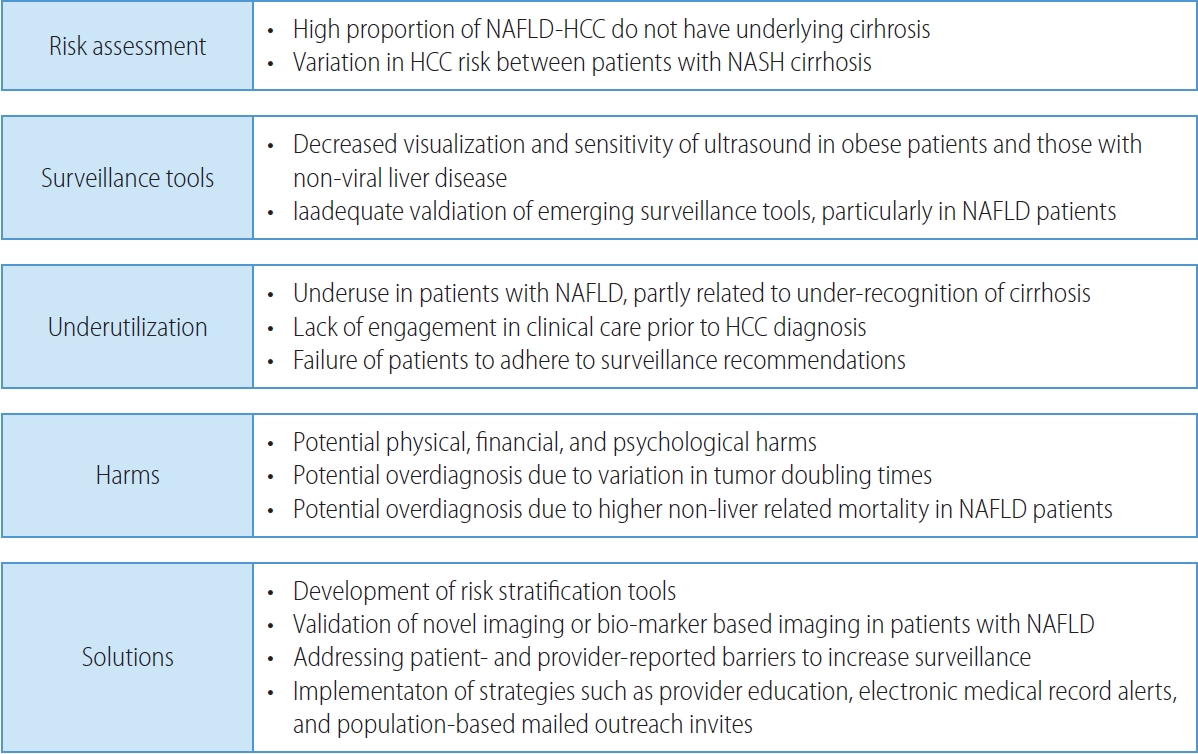
PDF - Non-alcoholic fatty liver disease (NAFLD), one of the most common causes of liver disease, is an increasingly common cause of hepatocellular carcinoma (HCC). Several demographic, clinical, and genetic factors contribute to HCC risk in NAFLD patients, which may inform risk stratification scores. Proven efficacious approaches to primary prevention approach in patients with non-viral liver disease remain an area of need. Semi-annual surveillance is associated with improved early tumor detection and reduced HCC-related mortality; however, patients with NAFLD have several challenges to effective surveillance, including under-recognition of at-risk patients, low surveillance utilization in clinical practice, and lower sensitivity of current tools for early-stage HCC detection. Treatment decisions are best made in a multidisciplinary fashion and are informed by several factors including tumor burden, liver dysfunction, performance status, and patient preferences. Although patients with NAFLD often have larger tumor burden and increased comorbidities compared to counterparts, they can achieve similar post-treatment survival with careful patient selection. Therefore, surgical therapies continue to provide a curative treatment option for patients diagnosed at an early stage. Although there has been debate about the efficacy of immune checkpoint inhibitors in patients with NAFLD, current data are insufficient to change treatment selection based on liver disease etiology.
-
Citations
Citations to this article as recorded by- Overnutrition and Lipotoxicity: Impaired Efferocytosis and Chronic Inflammation as Precursors to Multifaceted Disease Pathogenesis
Vivek Mann, Alamelu Sundaresan, Shishir Shishodia
Biology.2024; 13(4): 241. CrossRef - Risk of Hepatocellular Carcinoma by Steatotic Liver Disease and Its Newly Proposed Subclassification
Byeong Geun Song, Aryoung Kim, Myung Ji Goh, Wonseok Kang, Geum-Youn Gwak, Yong-Han Paik, Moon Seok Choi, Joon Hyeok Lee, Dong Hyun Sinn
Liver Cancer.2024; : 1. CrossRef - Risk of Bleeding in Hepatocellular Carcinoma Patients Treated with Atezolizumab/Bevacizumab: A Systematic Review and Meta-Analysis
Young-Gi Song, Kyeong-Min Yeom, Eun Ae Jung, Sang Gyune Kim, Young Seok Kim, Jeong-Ju Yoo
Liver Cancer.2024; : 1. CrossRef - Emerging role of exosomal microRNA in liver cancer in the era of precision medicine; potential and challenges
Tarek El Hayek, Osama Abdulwahab Alnaser-Almusa, Sulaiman Mamoun Alsalameh, Maya Taofik Alhalabi, Ahmad Nedal Sabbah, Eman Abdullah Alshehri, Tanveer Ahmad Mir, Naresh Kumar Mani, Khaled Al-Kattan, Raja Chinnappan, Ahmed Yaqinuddin
Frontiers in Molecular Biosciences.2024;[Epub] CrossRef - Trends in alcohol use and alcoholic liver disease in South Korea: a nationwide cohort study
Jeong-Ju Yoo, Dong Hyeon Lee, Young Chang, Hoongil Jo, Young Youn Cho, Sangheun Lee, Log Young Kim, Jae Young Jang
BMC Public Health.2024;[Epub] CrossRef - Smoking Increases the Risk of Hepatocellular Carcinoma and Cardiovascular Disease in Patients with Metabolic-Associated Fatty Liver Disease
Jeong-Ju Yoo, Man Young Park, Eun Ju Cho, Su Jong Yu, Sang Gyune Kim, Yoon Jun Kim, Young Seok Kim, Jung-Hwan Yoon
Journal of Clinical Medicine.2023; 12(9): 3336. CrossRef - Reply: Validation of MELD 3.0 scoring system in East Asian patients with cirrhosis awaiting liver transplantation
Jeong-Ju Yoo, Sang Gyune Kim
Liver Transplantation.2023; 29(11): E38. CrossRef - Unraveling the Janus-Faced Role of Autophagy in Hepatocellular Carcinoma: Implications for Therapeutic Interventions
Thi Ha Nguyen, Tuan Minh Nguyen, Dinh Thi Minh Ngoc, Taesik You, Mi Kyung Park, Chang Hoon Lee
International Journal of Molecular Sciences.2023; 24(22): 16255. CrossRef - Comparative Analysis of Atezolizumab Plus Bevacizumab and Hepatic Artery Infusion Chemotherapy in Unresectable Hepatocellular Carcinoma: A Multicenter, Propensity Score Study
Ji Kim, Hee-Chul Nam, Chang-Wook Kim, Hee Cho, Jae-Sung Yoo, Ji Han, Jeong Jang, Jong Choi, Seung Yoon, Hyun Yang, Si Bae, Suho Kim, Jung Oh, Ho Chun, Chang Jeon, Jaegyoon Ahn, Pil Sung
Cancers.2023; 15(17): 4233. CrossRef - A nationwide study on the current treatment status and natural prognosis of hepatocellular carcinoma in elderly
Jeong-Ju Yoo, Jayoun Lee, Gi Hong Choi, Min Woo Lee, Dong Ah Park
Scientific Reports.2023;[Epub] CrossRef
- Overnutrition and Lipotoxicity: Impaired Efferocytosis and Chronic Inflammation as Precursors to Multifaceted Disease Pathogenesis

Case Report
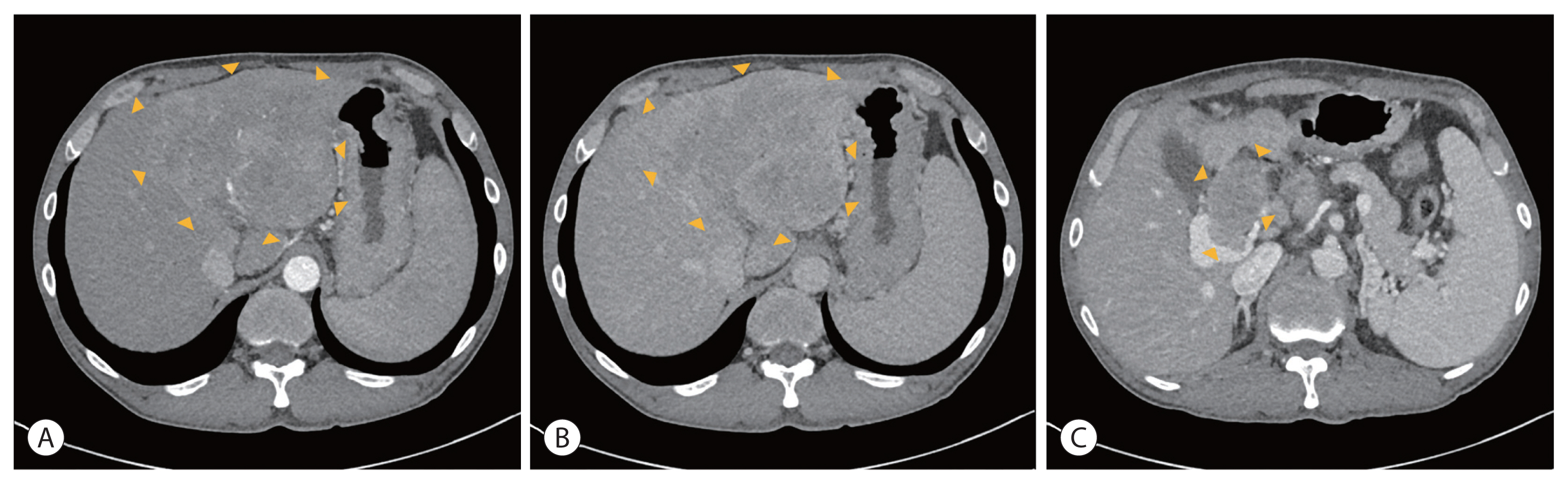
- Multidisciplinary treatment with immune checkpoint inhibitors for advanced stage hepatocellular carcinoma
- Ahlim Lee, Jaejun Lee, Hyun Yang, Soo-Yoon Sung, Chang Ho Jeon, Su Ho Kim, Moon Hyung Choi, Young Joon Lee, Ho Jong Chun, Si Hyun Bae
- J Liver Cancer. 2022;22(1):75-83. Published online March 18, 2022
- DOI: https://doi.org/10.17998/jlc.2022.03.04
- 4,464 Views
- 98 Downloads
- 4 Citations
-
 Abstract
Abstract
 PDF
PDF - Hepatocellular carcinoma (HCC) is a cytotoxic chemotherapy-resistant tumor and most HCCs arise in a background of liver cirrhosis of various causes. Although the IMBrave150 trial showed remarkable advancements in the treatment of unresectable HCC with atezolizumab plus bevacizumab (AteBeva), therapeutic outcomes were unsatisfactory in more than half of the patients. Accordingly, many ongoing trials combine conventional modalities with new drugs such as immune checkpoint inhibitors for better treatment outcomes, and they are expected to benefit patients with limited responses to conventional treatment. Here, two patients with advanced stage HCC with preserved liver function and good performance status showed partial response after treatment with combination or sequential therapy of AteBeva, hepatic arterial infusion chemotherapy, radiation therapy, and transarterial chemoembolization. These findings indicate the efficacy of multidisciplinary treatment against advanced HCC. Additional studies are required to establish optimal treatment strategies.
-
Citations
Citations to this article as recorded by- Complications of immunotherapy in advanced hepatocellular carcinoma
Young-Gi Song, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
Journal of Liver Cancer.2024; 24(1): 9. CrossRef - Higher objective responses by hepatic arterial infusion chemotherapy following atezolizumab and bevacizumab failure than when used as initial therapy in hepatocellular carcinoma: a retrospective study
Jae-Sung Yoo, Ji Hoon Kim, Hee Sun Cho, Ji Won Han, Jeong Won Jang, Jong Young Choi, Seung Kew Yoon, Suho Kim, Jung Suk Oh, Ho Jong Chun, Pil Soo Sung
Abdominal Radiology.2024;[Epub] CrossRef - Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab
Tae Hyun Kim, Bo Hyun Kim, Yu Ri Cho, Young-Hwan Koh, Joong-Won Park
Journal of Liver Cancer.2023; 23(2): 330. CrossRef - Is multidisciplinary treatment effective for hepatocellular carcinoma with portal vein tumor thrombus?
Won Hyeok Choe
Journal of Liver Cancer.2022; 22(1): 1. CrossRef
- Complications of immunotherapy in advanced hepatocellular carcinoma

Original Article
- Diagnostic performance of serum exosomal miRNA-720 in hepatocellular carcinoma
- Jeong Won Jang, Ji Min Kim, Hye Seon Kim, Jin Seoub Kim, Ji Won Han, Soon Kyu Lee, Heechul Nam, Pil Soo Sung, Si Hyun Bae, Jong Young Choi, Seung Kew Yoon
- J Liver Cancer. 2022;22(1):30-39. Published online March 21, 2022
- DOI: https://doi.org/10.17998/jlc.2022.02.25

- 4,408 Views
- 147 Downloads
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background/Aim
Hepatocellular carcinoma (HCC) is associated with poor prognosis, largely due to late detection. Highly accurate biomarkers are urgently needed to detect early-stage HCC. Our study aims to explore the diagnostic performance of serum exosomal microRNA (miR)-720 in HCC.
Methods
Exosomal miRNA was measured via quantitative real-time PCR. A correlation analysis of exosomal miR-720 and tumor or clinico-demographic data of patients with HCC was performed. The receiver operating characteristic (ROC) curve was used to assess the diagnostic capacity of serum exosomal miR-720 for HCC, in comparison with α-fetoprotein (AFP) and prothrombin induced by vitamin K absence or antagonist-II (PIVKA-II).
Results
MiR-720 was chosen as a potential HCC marker via miR microarray based on significant differential expression between tumor and non-tumor samples. Serum exosomal miR-720 was significantly upregulated in patients with HCC (n=114) versus other liver diseases (control, n=30), with a higher area under the ROC curve (AUC=0.931) than the other markers. Particularly, serum exosomal miR-720 showed superior performance in diagnosing small HCC (< 5 cm; AUC=0.930) compared with AFP (AUC=0.802) or PIVKA-II (AUC=0.718). Exosomal miR-720 levels showed marginal correlation with tumor size. The proportion of elevated miR-720 also increased with intrahepatic tumor stage progression. Unlike AFP or PIVKA-II showing a significant correlation with aminotransferase levels, the exosomal miR-720 level was not affected by aminotransferase levels.
Conclusions
Serum exosomal miR-720 is an excellent biomarker for the diagnosis of HCC, with better performance than AFP or PIVKA-II. Its diagnostic utility is maintained even in small HCC and is unaffected by aminotransferase levels. -
Citations
Citations to this article as recorded by- Prospects of liquid biopsy in the prognosis and clinical management of gastrointestinal cancers
Deepankar Mondal, Sapnita Shinde, Vibha Sinha, Vineeta Dixit, Souvik Paul, Rakesh Kumar Gupta, Suresh Thakur, Naveen Kumar Vishvakarma, Dhananjay Shukla
Frontiers in Molecular Biosciences.2024;[Epub] CrossRef - Emerging role of exosomal microRNA in liver cancer in the era of precision medicine; potential and challenges
Tarek El Hayek, Osama Abdulwahab Alnaser-Almusa, Sulaiman Mamoun Alsalameh, Maya Taofik Alhalabi, Ahmad Nedal Sabbah, Eman Abdullah Alshehri, Tanveer Ahmad Mir, Naresh Kumar Mani, Khaled Al-Kattan, Raja Chinnappan, Ahmed Yaqinuddin
Frontiers in Molecular Biosciences.2024;[Epub] CrossRef
- Prospects of liquid biopsy in the prognosis and clinical management of gastrointestinal cancers

Review Article
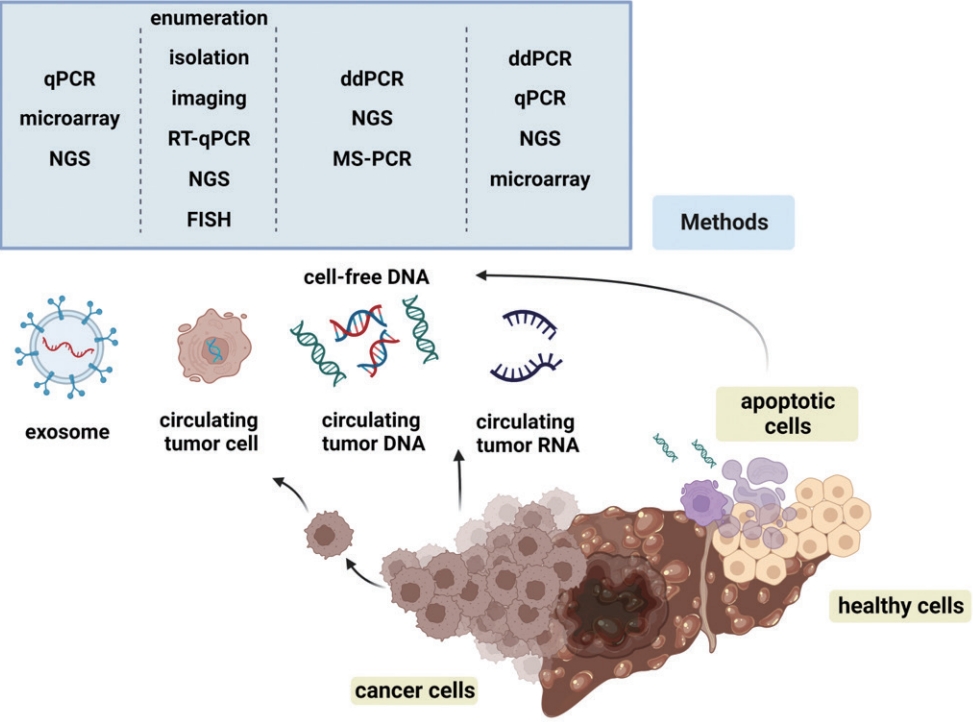
- Liquid biopsy for early detection and therapeutic monitoring of hepatocellular carcinoma
- Eun-Ji Choi, Young-Joon Kim
- J Liver Cancer. 2022;22(2):103-114. Published online September 22, 2022
- DOI: https://doi.org/10.17998/jlc.2022.09.08
- 4,252 Views
- 148 Downloads
- 6 Citations
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Advances in our knowledge of the molecular characteristics of hepatocellular carcinoma (HCC) have enabled significant progress in the detection and therapeutic prediction of HCC. As a non-invasive alternative to tissue biopsy, liquid biopsy examines circulating cellular components such as exosomes, nucleic acids, and cell-free DNA found in body fluids (e.g., urine, saliva, ascites, and pleural effusions) and provides information about tumor characteristics. Technical advances in liquid biopsy have led to the increasing adoption of diagnostic and monitoring applications for HCC. This review summarizes the various analytes, ongoing clinical trials, and case studies of United States Food and Drug Administrationapproved in vitro diagnostic applications for liquid biopsy, and provides insight into its implementation in managing HCC.
-
Citations
Citations to this article as recorded by- Biomarcatori tumorali: tra diagnostica clinica e medicina di precisione
Rossana FRANZIN
La Rivista Italiana della Medicina di Laboratorio.2024;[Epub] CrossRef - 16S rRNA Next-Generation Sequencing May Not Be Useful for Examining Suspected Cases of Spontaneous Bacterial Peritonitis
Chan Jin Yang, Ju Sun Song, Jeong-Ju Yoo, Keun Woo Park, Jina Yun, Sang Gyune Kim, Young Seok Kim
Medicina.2024; 60(2): 289. CrossRef - Korean urobiome platform (KUROM) study for acute uncomplicated sporadic versus recurrent cystitis in women: Clinical significance
Jeong-Ju Yoo, Hee Bong Shin, Ji Eun Moon, Sul Hee Lee, Hyemin Jeong, Hee Jo Yang, Woong Bin Kim, Kwang Woo Lee, Jae Heon Kim, Young Ho Kim
Investigative and Clinical Urology.2024; 65(4): 378. CrossRef - Current status of ultrasonography in national cancer surveillance program for hepatocellular carcinoma in South Korea: a large-scale multicenter study
Sun Hong Yoo, Soon Sun Kim, Sang Gyune Kim, Jung Hyun Kwon, Han-Ah Lee, Yeon Seok Seo, Young Kul Jung, Hyung Joon Yim, Do Seon Song, Seong Hee Kang, Moon Young Kim, Young-Hwan Ahn, Jieun Han, Young Seok Kim, Young Chang, Soung Won Jeong, Jae Young Jang, J
Journal of Liver Cancer.2023; 23(1): 189. CrossRef - Correspondence on Letter regarding “Long-term prognosis and the need for histologic assessment of chronic hepatitis B in the serological immune tolerant phase”
Jeong-Ju Yoo, Sang Gyune Kim
Clinical and Molecular Hepatology.2023; 29(2): 513. CrossRef - Exploring the Role of Circulating Cell-Free RNA in the Development of Colorectal Cancer
Chau-Ming Kan, Xiao Meng Pei, Martin Ho Yin Yeung, Nana Jin, Simon Siu Man Ng, Hin Fung Tsang, William Chi Shing Cho, Aldrin Kay-Yuen Yim, Allen Chi-Shing Yu, Sze Chuen Cesar Wong
International Journal of Molecular Sciences.2023; 24(13): 11026. CrossRef
- Biomarcatori tumorali: tra diagnostica clinica e medicina di precisione

Case Reports
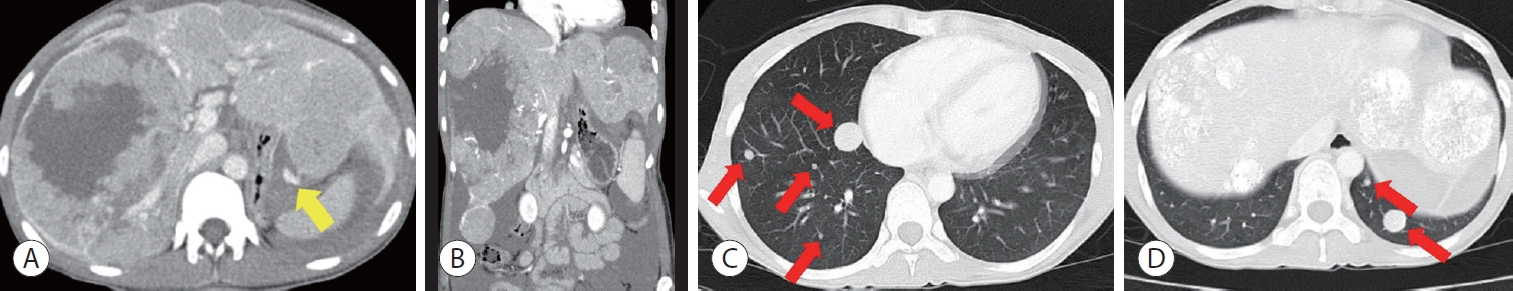
- A case report of advanced hepatocellular carcinoma treated with hepatic arterial infusion chemotherapy and sorafenib combination therapy followed by metastasectomy of lung and muscle metastases
- Sang Yi Moon, Sang Young Han, Yang-Hyun Baek
- J Liver Cancer. 2022;22(1):57-62. Published online January 6, 2022
- DOI: https://doi.org/10.17998/jlc.2021.12.20
- 3,816 Views
- 92 Downloads
-
 Abstract
Abstract
 PDF
PDF - Currently, various tyrosine kinase inhibitors and immune checkpoint inhibitors have been suggested in the treatment guidelines for advanced hepatocellular carcinoma (HCC). However, sorafenib was the only systemic drug approved 10 years ago. In 2010, a woman diagnosed with HCC rupture and multiple lung metastases visited our hospital. At the time of visiting our hospital, she had undergone transarterial chemoembolization at another hospital to control bleeding due to HCC rupture. We treated her with hepatic arterial infusion chemotherapy and sorafenib combination therapy to increase the control of intrahepatic tumors in consideration of the modest efficacy of sorafenib. The intrahepatic tumor was almost controlled. Metastasectomy was performed to control lung oligometastasis. Subsequently, additional muscle metastasis was confirmed, and metastasectomy was performed. Although this is a very rare case, it shows that a multidisciplinary approach can improve the prognosis of patients with HCC.

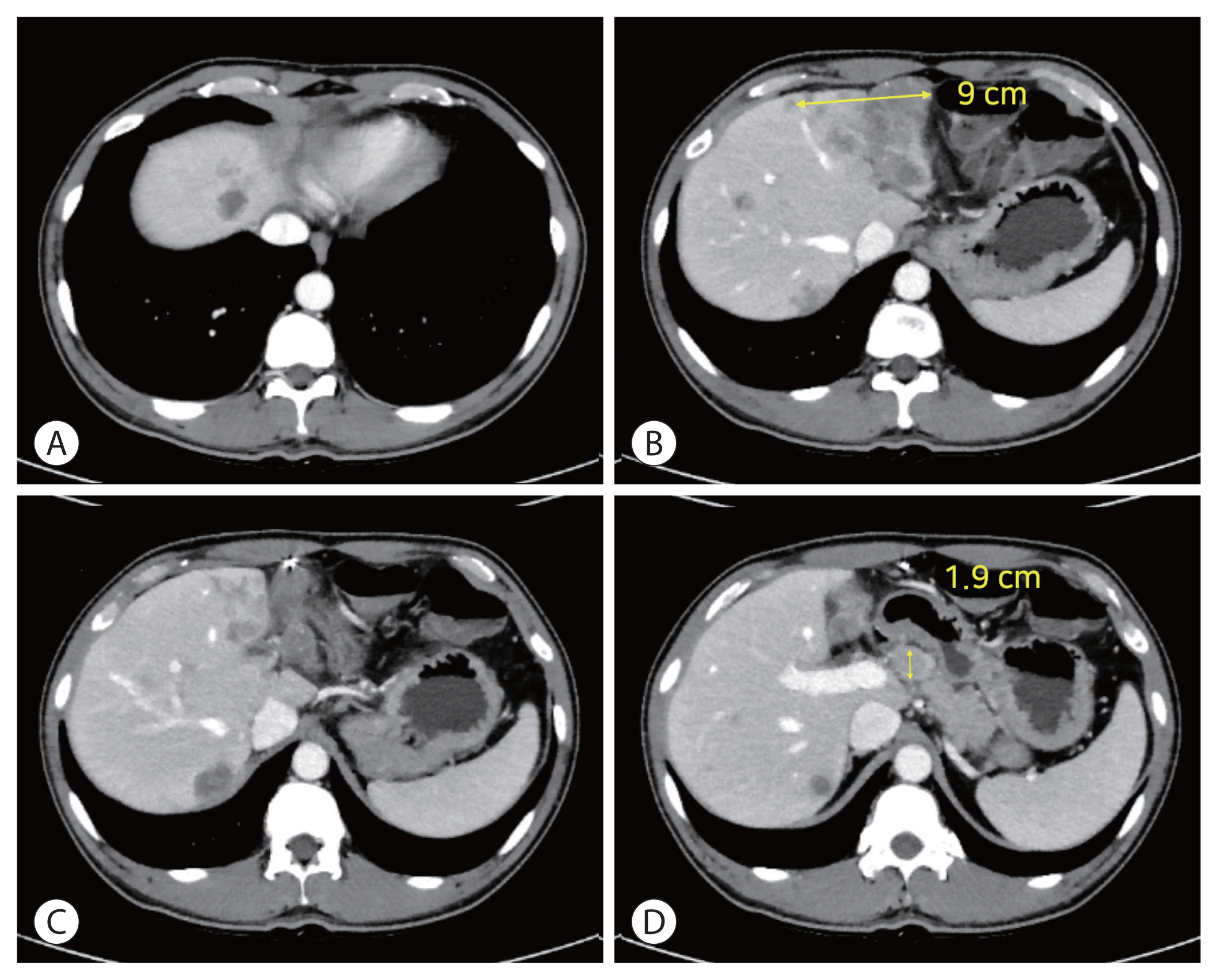
- Concurrent transarterial radioembolization and combination atezolizumab/ bevacizumab treatment of infiltrative hepatocellular carcinoma with portal vein tumor thrombosis: a case report
- Min Kyung Park, Su Jong Yu
- J Liver Cancer. 2022;22(1):69-74. Published online March 21, 2022
- DOI: https://doi.org/10.17998/jlc.2022.03.09
- 3,745 Views
- 112 Downloads
- 4 Citations
-
 Abstract
Abstract
 PDF
PDF - Treatment options for advanced hepatocellular carcinoma (HCC) have been rapidly evolving. Herein, we describe a patient with advanced HCC and portal vein tumor thrombosis (PVTT) who responded decisively to a multidisciplinary approach. The patient had an ill-defined infiltrative HCC (diffuse subtype), with several intrahepatic metastasis and tumor invasion of left portal vein. Concurrent use of transarterial radioembolization (TARE) and systemic therapeutics (atezolizumab + bevacizumab) ultimately proved successful. There was marked reduction in tumor volume after TARE and an additional three cycles of atezolizumab plus bevacizumab. This concurrent treatment was well tolerated, without adverse events during immunotherapy. The impressive results achieved suggest that concurrent TARE and combination atezolizumab/bevacizumab is a promising treatment approach for advanced HCC with PVTT.
-
Citations
Citations to this article as recorded by- Biologics, Immunotherapies, and Cytotoxic Chemotherapy for Hepatocellular Carcinoma following Current Recommendations by the BCLC: A Review of Agents
Rajangad S. Gurtatta, Sydney E. Whalen, Charles E. Ray
Seminars in Interventional Radiology.2024; 41(01): 084. CrossRef - Combining immunotherapy with transarterial radioembolization
ZeynepCeren Balaban Genc, Efe Soydemır, SevalAy Ersoy, Tunc Ones
Indian Journal of Nuclear Medicine.2023; 38(2): 145. CrossRef - The New Era of Systemic Treatment for Hepatocellular Carcinoma: From the First Line to the Optimal Sequence
Maria Cerreto, Ferdinando Cardone, Lucia Cerrito, Leonardo Stella, Francesco Santopaolo, Maria Pallozzi, Antonio Gasbarrini, Francesca Romana Ponziani
Current Oncology.2023; 30(10): 8774. CrossRef - Is multidisciplinary treatment effective for hepatocellular carcinoma with portal vein tumor thrombus?
Won Hyeok Choe
Journal of Liver Cancer.2022; 22(1): 1. CrossRef
- Biologics, Immunotherapies, and Cytotoxic Chemotherapy for Hepatocellular Carcinoma following Current Recommendations by the BCLC: A Review of Agents

Original Article
- The effects of immune checkpoint modulators on the clinical course of patients with resectable hepatocellular carcinoma
- Jihyun An, Hyo Jeong Kang, Eunsil Yu, Han Chu Lee, Ju Hyun Shim
- J Liver Cancer. 2022;22(1):40-50. Published online March 17, 2022
- DOI: https://doi.org/10.17998/jlc.2022.03.06

- 3,735 Views
- 121 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background/Aim
Immune checkpoint proteins regulating T-cell mediated anti-tumor immunity have been reported to affect clinical outcomes in multiple malignancies. This study aimed to investigate the prognostic effect of histological expression of immune checkpoint proteins in patients with resected hepatocellular carcinoma (HCC).
Methods
A total of 221 patients with HCC who underwent curative resection were included. Expression of programmed-cell death ligand-1 (PD-L1) in tumor cells (tPD-L1) and tumor infiltrating mononuclear cells (TIMCs) (iPD-L1), programmed-cell death-1 in TIMCs (iPD-1), and cytotoxic T lymphocyte antigen-4 in TIMCs (iCTLA-4) were measured immunohistochemically.
Results
Histo-positivity for iCTLA-4, iPD-1, iPD-L1, and tPD-L1 was 32.1%, 42.5%, 35.3%, and 14.9%, respectively. Multivariate logistic analyses revealed that male sex and tumor >5 cm were variables related to iCTLA-4 positivity (odds ratio [OR], 0.46 and 1.94, respectively; P<0.05). Poor differentiation was related to PD-L1 expression in both tumor cells and TIMCs (OR, 2.88 and 3.46, respectively; P<0.05). Microvascular invasion was significantly associated only with iPD-L1 (OR, 2.24; P<0.05). In time-dependent outcome analyses, expression of immune checkpoint proteins in TIMCs (i.e., iCTLA-4, iPD-1, and iPD-L1) was significantly related to longer overall survival and non-cancer-related survival (all P<0.05), but not to time-to-recurrence or cancer-specific deaths. Concurrent activation of the PD-1:PD-L1 and CTLA-4 pathways predicted improved outcomes in terms of overall survival and non-cancer related survival (P=0.06 and P=0.03, respectively).
Conclusions
Immune checkpoint proteins upregulated in TIMCs in HCC tissues have individual and additive effects in prolonging the survival of patients, specifically in terms of survival not related to cancer recurrence. -
Citations
Citations to this article as recorded by- The role of PD-1/PD-L1 signaling pathway in cancer pathogenesis and treatment: a systematic review
Amirhosein Sabaghian, Shahnam Shamsabadi, Saghar Momeni, Mobina Mohammadikia, Kiarash Mohebbipour, Samira Sanami, Sajjad Ahmad, Nahid Akhtar, Neeta Raj Sharma, Raja Babu Singh Kushwah, Yash Gupta, Ajit Prakash, Hamidreza Pazoki-Toroudi
Journal of Cancer Metastasis and Treatment.2024;[Epub] CrossRef
- The role of PD-1/PD-L1 signaling pathway in cancer pathogenesis and treatment: a systematic review

Review Article
- The role of lenvatinib in the era of immunotherapy of hepatocellular carcinoma
- Matthew Man Pok Lee, Landon Long Chan, Stephen Lam Chan
- J Liver Cancer. 2023;23(2):262-271. Published online August 17, 2023
- DOI: https://doi.org/10.17998/jlc.2023.07.17
- 3,532 Views
- 281 Downloads
- 5 Citations
-
 Abstract
Abstract
 PDF
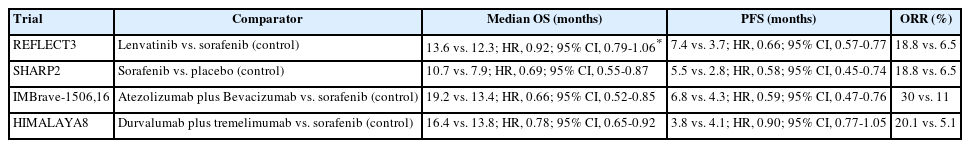
PDF - Hepatocellular carcinoma (HCC) frequently presents as advanced stage with poor prognosis and high mortality. Systemic treatment is the treatment of choice for advanced disease. In 2007, the first multi-kinase inhibitor (MKI) sorafenib was approved and shown to modestly prolong overall survival (OS). The progress of systemic therapy has been slow afterwards until 2018 when lenvatinib, another MKI, was shown to be non-inferior to sorafenib on median OS as the first-line therapy for HCC. Since then, remarkable progress has been achieved on the treatment of advanced HCC, including the development of second-line targeted treatment, including regorafenib, cabozantinib and ramucirumab from 2017 to 2019. A growing focus has been placed on immune checkpoint inhibitors (ICIs) targeting programmed cell death-1 (PD-1), its ligand PD-L1, and cytotoxic T-lymphocyte-associated protein 4. These ICIs have proven their potency in treating HCC as both initial and subsequent line of therapy. At present, both regimens of atezolizumab combined with bevacizumab, as well as the combination of tremelimumab and durvalumab, are recommended as the first-line treatments based on positive phase III clinical trials. With the advancement of ICIs, it is anticipated that the role of MKIs in the treatment of HCC will evolve. In this article, lenvatinib, one of the most commonly used MKIs in HCC, is chosen to be reviewed.
-
Citations
Citations to this article as recorded by- Reduced-Dose or Discontinuation of Bevacizumab Might Be Considered after Variceal Bleeding in Patients with Hepatocellular Carcinoma Receiving Atezolizumab/Bevacizumab: Case Reports
Kyeong-Min Yeom, Young-Gi Song, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
Medicina.2024; 60(1): 157. CrossRef - The Position of Multikinase Inhibitors in the Era of Immune-Checkpoint Inhibitors for Hepatocellular Carcinoma
Beom Kyung Kim
Gut and Liver.2024; 18(1): 3. CrossRef - Fatal intratumoral hemorrhage in a patient with hepatocellular carcinoma following successful treatment with atezolizumab/bevacizumab: A case report
Kyeong-Hoon Park, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
World Journal of Clinical Cases.2024; 12(22): 5177. CrossRef - Small molecule tyrosine kinase inhibitors approved for systemic therapy of advanced hepatocellular carcinoma: recent advances and future perspectives
Jianzhong Liu, Shuai Xia, Baoyi Zhang, Dina Mostafa Mohammed, Xiangliang Yang, Yanhong Zhu, Xinnong Jiang
Discover Oncology.2024;[Epub] CrossRef - Consistent efficacy of hepatic artery infusion chemotherapy irrespective of PD‑L1 positivity in unresectable hepatocellular carcinoma
Ji Kim, Young Kim, Hee-Chul Nam, Chang-Wook Kim, Jae-Sung Yoo, Ji Han, Jeong Jang, Jong Choi, Seung Yoon, Ho Jong Chun, Jung Oh, Suho Kim, Sung Lee, Pil Sung
Oncology Letters.2024;[Epub] CrossRef
- Reduced-Dose or Discontinuation of Bevacizumab Might Be Considered after Variceal Bleeding in Patients with Hepatocellular Carcinoma Receiving Atezolizumab/Bevacizumab: Case Reports

Original Article
- Effect of direct-acting antivirals for hepatitis C virus-related hepatocellular carcinoma recurrence and death after curative treatment
- Young-Hwan Ahn, Heirim Lee, Ji Eun Han, Hyo Jung Cho, Jae Youn Cheong, Bumhee Park, Soon Sun Kim
- J Liver Cancer. 2022;22(2):125-135. Published online June 28, 2022
- DOI: https://doi.org/10.17998/jlc.2022.05.24
- 3,530 Views
- 86 Downloads
- 4 Citations
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background/Aim
There has been a long-standing debate about the association of directacting antiviral (DAA) therapy and hepatocellular carcinoma (HCC) recurrence. This study aimed to investigate the association between DAA therapy and HCC recurrence after curative therapy.
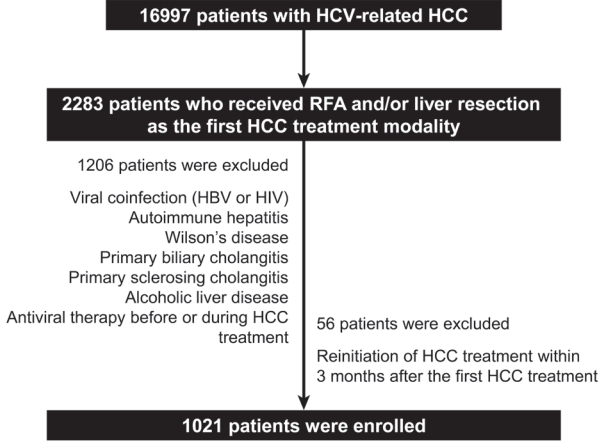
Methods
We retrospectively enrolled 1,021 patients with HCV-related (hepatitis C virus) HCC who underwent radiofrequency ablation (RFA), liver resection, or both as the first treatment modality from January 2007 to December 2016 and without a history of HCV therapy before HCC treatment from a nationwide database. The effect of HCV treatment on HCC recurrence and all-cause mortality was also investigated.
Results
Among the 1,021 patients, 77 (7.5%) were treated with DAA, 14 (1.4%) were treated with interferon-based therapy, and 930 (91.1%) did not receive HCV therapy. DAA therapy was an independent prognostic factor for lower HCC recurrence rate (hazard ratio [HR], 0.04; 95% confidence interval [CI], 0.006-0.289; P=0.001 for landmarks at 6 months after HCC treatment and HR, 0.05; 95% CI, 0.007-0.354; P=0.003 for landmarks at 1 year). Furthermore, DAA therapy was associated with lower all-cause mortality (HR, 0.049; 95% CI, 0.007-0.349; P=0.003 for landmarks at 6 months and HR, 0.063; 95% CI, 0.009-0.451; P=0.006 for landmarks at 1 year).
Conclusions
DAA therapy after curative HCC treatment can decrease HCC recurrence and all-cause mortality compared to interferon-based therapy or no antiviral therapy. Therefore, clinicians should consider administering DAA therapy after curative HCC treatment in patients with HCV-related HCC. -
Citations
Citations to this article as recorded by- Comparison of Surgical Resection and Radiofrequency Ablation in Elderly Patients with Hepatocellular Carcinoma
Jun Il Kim, Jayoun Lee, Gi Hong Choi, Min Woo Lee, Dong Ah Park, Jeong-Ju Yoo
Digestive Diseases and Sciences.2024; 69(3): 1055. CrossRef - Analyzing risk factors and developing a stratification system for hepatocellular carcinoma recurrence after interferon-free direct-acting antiviral therapy in chronic hepatitis C patients
Chih-Hsuan Luan, Pin-Shuo Su, Chi-Jen Chu, Chung-Chi Lin, Chien-Wei Su, Jiing-Chyuan Luo, I-Cheng Lee, Chen-Ta Chi, Shou-Dong Lee, Yuan-Jen Wang, Fa-Yauh Lee, Yi-Hsiang Huang, Ming-Chih Hou
Journal of the Chinese Medical Association.2024; 87(4): 357. CrossRef - Addition of Kidney Dysfunction Type to MELD-Na for the Prediction of Survival in Cirrhotic Patients Awaiting Liver Transplantation in Comparison with MELD 3.0 with Albumin
Kyeong-Min Yeom, Jong-In Chang, Jeong-Ju Yoo, Ji Eun Moon, Dong Hyun Sinn, Young Seok Kim, Sang Gyune Kim
Diagnostics.2023; 14(1): 39. CrossRef - Is direct-acting antiviral treatment beneficial or harmful for patients with hepatitis C virus-related hepatocellular carcinoma?
Hye Won Lee
Journal of Liver Cancer.2022; 22(2): 91. CrossRef
- Comparison of Surgical Resection and Radiofrequency Ablation in Elderly Patients with Hepatocellular Carcinoma

Case Report
- Complete response to local therapy for advanced hepatocellular carcinoma with lung metastasis: a case report
- Daeun Kim, Seiyeon Park, Won Sohn, Hyun Pyo Hong, Byung Ik Kim
- J Liver Cancer. 2022;22(1):51-56. Published online January 27, 2022
- DOI: https://doi.org/10.17998/jlc.2021.12.28
- 3,491 Views
- 104 Downloads
-
 Abstract
Abstract
 PDF
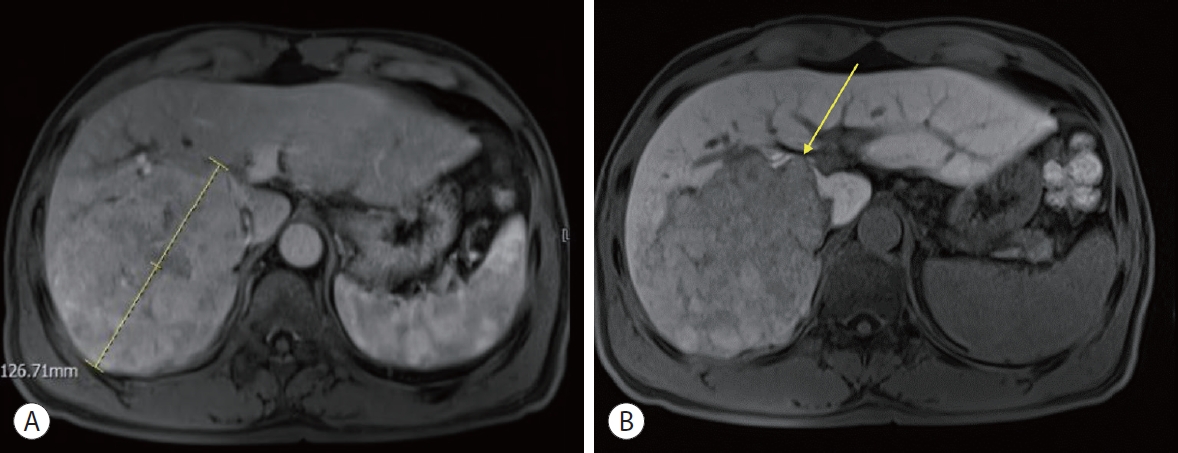
PDF - The concept of oligometastasis is widely accepted for various types of solid tumors; accordingly, better outcomes can be anticipated with aggressive local interventions. The treatment of advanced hepatocellular carcinoma (HCC) with extrahepatic metastasis is systemic therapy. However, treatment responses to systemic therapy are poor. Recently, a small number of metastatic cancers (oligometastasis) have been controlled by local therapy rather than systemic therapy. Our study reports a case of a 66-year-old male patient with advanced HCC with lung metastasis, which was treated with local therapy. There were less than four metastases in the lungs, which were treated with wedge resection, radiofrequency, and radiation therapy. He repeatedly underwent local therapy for lung oligometastasis and locoregional therapy for intrahepatic HCC rather than systemic therapy; control by local therapy was possible as his liver function was preserved with Child-Turcotte-Pugh class A.


 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION

 First
First Prev
Prev



 Follow JLC on Twitter
Follow JLC on Twitter