Ahead-of-print articles
- Page Path
- HOME > Articles and issues > Ahead-of-print articles
Articles in E-pub version are posted online ahead of regular printed publication.
Original Articles
- Recent update of proton beam therapy for hepatocellular carcinoma: A systematic review and meta-analysis
- Sun Hyun Bae, Won Il Jang, Hanna Rahbek Mortensen, Britta Weber, Mi Sook Kim, Morten Høyer
- Received May 16, 2024 Accepted June 26, 2024 Published online July 4, 2024
- DOI: https://doi.org/10.17998/jlc.2024.06.26 [Accepted]
- 420 Views
- 16 Downloads
-
 Abstract
Abstract
 PDF
PDF - Backgrounds/Aims
Although access to proton beam therapy (PBT) is limited worldwide, its use for the treatment of hepatocellular carcinoma (HCC) is gradually increasing with the expansion of new facilities. Therefore, we conducted a systematic review and meta-analysis to investigate the updated evidence of PBT for HCC.
Methods
The MEDLINE, EMBASE, Cochrane Library, and Web of Science databases were systematically searched for studies that enrolled patients with liver-confined HCC that were treated with PBT for a cure up to February 2024.
Results
A total of 1858 HCC patients receiving PBT from 22 studies between 2004 and 2023 were selected for this meta-analysis. The median proportion of Child-Pugh class A was 86% (range: 41–100%), and the median tumor size was 3.6 cm (range: 1.2–9 cm). The median total dose ranged from 55 GyE to 76 GyE (median, 69 GyE). The pooled rates of 3- and 5-year local progression-free survival after PBT were 88% (95% confidence interval [CI], 85–91%) and 86% (95% CI, 82–90%), respectively. The pooled 3- and 5-year overall rates were 60% (95% CI, 54–66%) and 46% (95% CI, 38–54%), respectively. The pooled rates of grade 3 hepatic toxicity, classic radiation-induced liver disease (RILD), and non-classic RILD were 1%, 2%, and 1%, respectively.
Conclusions
The current study supports PBT for HCC and demonstrates favorable long-term survival and low hepatic toxicities compared with other published studies on other radiotherapy modalities. However, further studies are needed to identify the subgroups that will benefit from PBT.

- Assessment of Real-Time US-CT/MR-guided Percutaneous Gold Fiducial Marker Implementation in Malignant Hepatic Tumors for Stereotactic Body Radiation Therapy
- Sungjun Hwang, Seok-Joo Chun, Eui Kyu Chie, Jeong Min Lee
- Received March 20, 2024 Accepted June 2, 2024 Published online June 10, 2024
- DOI: https://doi.org/10.17998/jlc.2024.06.03 [Accepted]

- 482 Views
- 13 Downloads
-
 Abstract
Abstract
 PDF
PDF - Background/Aims
This study explored the initial institutional experience of using gold fiducial markers for stereotactic body radiotherapy (SBRT) in treating malignant hepatic tumors using real-time ultrasound-computed tomography (CT)/magnetic resonance (MR) imaging fusion-guided percutaneous placement.
Methods
From May 2021 to August 2023, 19 patients with 25 liver tumors that were invisible on pre-contrast CT received fiducial markers following these guidelines. Postprocedural scans were used to confirm their placement. We assessed technical and clinical success rates and monitored complications. The implantation of fiducial markers facilitating adequate treatment prior to SBRT, which was achieved in 96% of the cases (24 of 25 tumors), was considered technical success. Clinical success was the successful completion of SBRT without evidence of marker displacement and was achieved in 88% of cases (22 of 25 tumors). Complications included one major subcapsular hematoma and marker migration into the right atrium in two cases, which prevented SBRT.
Results
Among the treated tumors, 83.3% (20 of 24) showed a complete response, 12.5% (3 of 24) remained stable, and 4.2% (1 of 24) progressed during an average 11.7-month follow-up (range, 2–32 months).
Conclusions
This study confirms that percutaneous gold fiducial marker placement using real-time CT/MR guidance is effective and safe for SBRT in hepatic tumors, but warns of marker migration risks, especially near the hepatic veins and in subcapsular locations. Using fewer markers than traditionally recommended—typically two per patient), the outcomes were still satisfactory, particularly given the increased risk of migration when markers were placed near major hepatic veins.

- Heavy Smoking Increases Early Mortality Risk in Patients with Hepatocellular Carcinoma after Curative Treatment
- Jaejun Lee, Jong Young Choi, Soon Kyu Lee
- Received April 24, 2024 Accepted June 2, 2024 Published online June 7, 2024
- DOI: https://doi.org/10.17998/jlc.2024.06.02 [Accepted]

- 582 Views
- 31 Downloads
-
 Abstract
Abstract
 PDF
PDF - Background
Although cigarette smoking has been associated with an increased risk of hepatocellular carcinoma (HCC), its association with HCC mortality remains underexplored. We aimed to evaluate the effect of smoking on early mortality in HCC patients following curative treatment.
Methods
Data from the Korean Primary Liver Cancer Registry were examined for HCC patients who underwent liver resection or radiofrequency ablation between 2015 and 2018. Smoking cumulative dose was assessed in pack-years. The primary outcome was the 3-year overall survival (OS).
Results
Among 1924 patients, 161 were classified as heavy smokers (≥ 40 pack-years). Heavy smokers exhibited a lower 3-year survival rate (77.1 %) than nonsmokers (83.3%), with a significant difference observed in the 3-year OS (p = 0.016). The assessment of smoking packyears in relation to 3-year OS revealed a dose-dependent pattern, with the hazard ratio exceeding 1.0 at 20 pack-years and continuing to rise until 40 pack-years, reaching peak at 1.21 (95% confidence interval: 1.01, 1.45). Multivariate Cox-regression analysis revealed heavy smoking, age ≥ 60 y, underlying cirrhosis, tumor size > 3 cm, vascular invasion, and Child-Pugh class B/C as risk factors for 3-year OS. Subgroup analyses of patients with a tumor size < 3 cm, absence of vascular invasion, and meeting the Milan criteria also showed inferior outcomes for heavy smokers in all three subgroups.
Conclusion
Heavy smoking, defined as a history of > 40 pack-years, was linked to poorer 3-year survival outcomes in HCC patients undergoing curative treatments, underscoring the importance of smoking cessation in this population.

- Role of transarterial chemoembolization for hepatocellular carcinoma with extrahepatic metastases in the era of advancing systemic therapy
- Byeong Geun Song, Myung Ji Goh, Wonseok Kang, Dong Hyun Sinn, Geum-Youn Gwak, Yong-Han Paik, Joon Hyeok Lee, Moon Seok Choi
- Received March 5, 2024 Accepted May 26, 2024 Published online June 3, 2024
- DOI: https://doi.org/10.17998/jlc.2024.05.26 [Accepted]

- 614 Views
- 33 Downloads
-
 Abstract
Abstract
 PDF
PDF - Background/Aims
Systemic therapy is the current standard treatment for hepatocellular carcinoma (HCC) with extrahepatic metastases (EHM). However, some patients with HCC and EHM undergo transarterial chemoembolization (TACE) to manage intrahepatic tumors. Herein, we aimed to explore the appropriateness of TACE in patients with HCC and EHM in an era of advanced systemic therapy.
Methods
This study analyzed 248 consecutive patients with HCC and EHM (median age 58.5 years, 83.5% male, and 88.7% Child-Pugh A) who received TACE or systemic therapy (83 sorafenib, 49 lenvatinib, 28 immunotherapy-based) between January 2018 and January 2021.
Results
Among the patients, 196 deaths were recorded during a median follow-up of 8.9 months. Patients who received systemic therapy had a higher albumin-bilirubin grade, elevated tumor markers, an increased number of intrahepatic tumors, larger-sized tumors, and more frequent portal vein invasion than those who underwent TACE. TACE was associated with longer median overall survival (OS) than sorafenib (15.1 vs. 4.7 months; 95% confidence interval [CI]: 11.1–22.2 vs. 3.7–7.3; hazard ratio [HR] 1.97, P<0.001). After adjustment for potential confounders, TACE was associated with statistically similar survival outcomes to those of lenvatinib (median OS: 8.0 months; 95% CI: 6.5–11.0; HR 1.21, P=0.411) and immunotherapies (median OS: 14.3 months; 95% CI: 9.5–27.0; HR 1.01, P=0.973), demonstrating survival benefits equivalent to these treatments.
Conclusion
In patients with HCC and EHM, TACE can provide a survival benefit comparable to that of newer systemic therapies. Accordingly, TACE remains a valuable option in this era of new systemic therapies.

- Cure can be Achieved by Conversion to Microwave Ablation following Atezolizumab-Bevacizumab Therapy in Unresectable Hepatocellular Carcinoma
- Rene John D. Febro, Engelbert Simon S. Perillo, Akemi A. Kimura, Stephen N. Wong
- Received February 19, 2024 Accepted May 23, 2024 Published online June 3, 2024
- DOI: https://doi.org/10.17998/jlc.2024.05.23 [Accepted]

- 1,382 Views
- 66 Downloads
-
 Abstract
Abstract
 PDF
PDF - Introduction
Atezolizumab/bevacizumab is the recommended first-line systemic therapy for unresectable hepatocellular carcinoma (uHCC) and may facilitate curative conversion through resection and locoregional therapies. However, there have been very few reports on curative conversion using microwave ablation (MWA). This study aimed to determine the curative conversion rate with MWA using atezolizumab-bevacizumab as the first-line treatment in patients with uHCC, and to compare the characteristics and survival of patients with and without curative conversion.
Methods
Consecutive patients with uHCC who were started on atezolizumab-bevacizumab from May 2021 and December 2023 in a single tertiary center were included. Objective response (ORR) and disease control rate (DCR) were based on the RECIST 1.1 and mRECIST criteria.
Results
Twenty consecutive patients with uHCC (60% advanced-stage) were included, 90% exceeding the up-to-7 criteria. The ORR and DCR were 35% and 60%, and 35% and 55% using RECIST and mRECIST, respectively. Five (25%) patients underwent successful curative conversion with MWA (4 advanced and 1 intermediate stage) despite a median HCC size of 6.1 (range: 2.4-7.3) cm. Two of these patients were tumor and drug-free 132-133 weeks from the 1st atezolizumab-bevacizumab dose. Patients who underwent curative conversion had significantly longer survival than those who did not. (p=0.024) Other factors associated with survival were male sex, Child-Pugh class A, and an objective response.
Conclusions
Despite the relatively large tumor size, successful curative conversion with MWA was achieved with first-line atezolizumab-bevacizumab in uHCC. However, data from prospective multicenter trials are required to determine whether this strategy is universally applicable.

- Downstaging with Atezolizumab-Bevacizumab: A Case Series
- Anand V. Kulkarni, Kumaraswamy P, Balachandran Menon, Anuradha Sekaran, Anuhya Rambhatl, Sowmya Iyengar, Manasa Alla, Shantan Venishetty, Sumana Kolar Ramachandra, Premkumar G V, Mithun Sharma, P. N Rao, Duvvur Nageshwar Reddy, Amit G. Singal
- Received April 3, 2024 Accepted May 12, 2024 Published online May 27, 2024
- DOI: https://doi.org/10.17998/jlc.2024.05.12 [Accepted]

- 1,047 Views
- 140 Downloads
-
 Abstract
Abstract
 PDF
PDF - Background/aims
Hepatocellular carcinoma (HCC) is generally diagnosed at an advanced stage, which limits curative treatment options for these patients. Locoregional therapy (LRT) is the standard approach to bridge and downstage unresectable HCC (uHCC) for liver transplantation (LT). Atezolizumab-bevacizumab (atezo-bev) can induce objective responses in nearly one-third of patients; however, the role and outcomes of downstaging using atezobev remains unknown.
Methods
In this retrospective single-center study, we included consecutive patients between November 2020 and August 2023, who received atezo-bev with or without LRT and were subsequently considered for resection/LT after downstaging.
Results
Of the 115 patients who received atezo-bev, 12 patients (10.4%) achieved complete or partial response and were willing to undergo LT; they (age: 58.5 years; women-17%; Barcelona Clinic Liver Cancer Stage System B/C:5/7) had received 3–12 cycles of atezo-bev, and 4 of them had received prior LRT. Three patients died before LT, while three were awaiting LT. Six patients underwent curative therapies: four underwent living donor LT after a median of 79.5 (54–114) days following the last atezo-bev dose, one underwent deceased donor LT 38 days after the last dose, and one underwent resection. All but one patient had complete pathologic response with no viable HCC. Three patients experienced wound healing complications, and one required re-exploration and succumbed to sepsis. After a median follow-up of 10 (4–30) months, none of the alive patients developed HCC recurrence or graft rejection.
Conclusions
Surgical therapy, including LT, is possible after atezo-bev therapy in wellselected patients after downstaging.

- Superselective Ablative Chemo-ethanol Embolization for Recurrent Single Hepatocellular Carcinoma: A Six-Month Outcome Analysis
- Jae Hwan Lee, Kun Yung Kim, Chong-ho Lee, Minuk Kim, Chang Jin Yoon
- Received April 1, 2024 Accepted May 8, 2024 Published online May 14, 2024
- DOI: https://doi.org/10.17998/jlc.2024.05.08 [Accepted]

- 456 Views
- 29 Downloads
-
 Abstract
Abstract
 PDF
PDF - Background/Aim
To evaluate the safety and effectiveness of superselective ablative chemoethanol embolization (SACE) for the treatment of patients with recurrent single hepatocellular carcinoma (rHCC).
Materials and Methods
This retrospective study included 22 patients (19 men, median age 63 [range 38-86 y]) with Child-Pugh class of A/B/C (16/3/3) that underwent SACE between January and June 2023 for recurrent single HCCs measuring ≤ 5 cm in diameter using a mixture of 99% Ethanol and ethiodized oil/doxorubicin emulsion. The primary endpoint was the 6-month tumor response, and the secondary endpoints were the 1-month tumor response and treatment-related safety. This study was approved by our institutional review board, and the requirement for informed consent was waived.
Results
SACE was successfully performed in 22 (95.2%) patients. The complete response rates at 1-month and 6-month after treatment were 100% and 83.3%, respectively. At 6-month, local tumor progression occurred in one patient and intrahepatic distant metastasis was found in 6 (30%) patients. No 6-month mortalities were reported. No adverse events greater than grade 2 or laboratory deteriorations were observed. Biliary complications or liver abscesses were not observed.
Conclusion
SACE for a single rHCC was highly effective in achieving a favorable 6-month tumor response and showed acceptable adverse events. However, further prospective studies are required to verify these findings.

- Re-assessing the diagnostic value of the enhancing “capsule” in hepatocellular carcinoma imaging
- Jae Seok Bae, Jeong Min Lee, Bo Yun Hur, Jeongin Yoo, Sae-Jin Park
- Received March 8, 2024 Accepted May 1, 2024 Published online May 8, 2024
- DOI: https://doi.org/10.17998/jlc.2024.05.01 [Accepted]

- 1,072 Views
- 19 Downloads
-
 Abstract
Abstract
 PDF
PDF - Background/Aims
The enhancing “capsule” (EC) in hepatocellular carcinoma (HCC) diagnosis has received varying degrees of recognition across major guidelines. This study aimed to assess the diagnostic utility of EC in HCC detection.
Methods
We retrospectively analyzed patients who underwent pre-surgical computed tomography (CT) and hepatobiliary agent-enhanced magnetic resonance imaging (HBA-MRI) between January 2016 and December 2019. A single hepatic tumor was confirmed based on the pathology of each patient. Three radiologists independently reviewed the images according to the Liver Imaging Reporting and Data System (LIRADS) v2018 criteria and reached a consensus. Interobserver agreement for EC before reaching a consensus was quantified using Fleiss κ statistics. The impact of EC on the LI-RADS classification was assessed by comparing the positive predictive values for HCC detection in the presence and absence of EC.
Results
In total, 237 patients (median age, 60 years; 184 men) with 237 observations were included. The interobserver agreement for EC detection was notably low for CT (κ=0.169) and HBA-MRI (κ=0.138). The presence of EC did not significantly alter the positive predictive value for HCC detection in LI-RADS category 5 observations on CT (94.1% [80/85] vs. 94.6% [88/93], P=0.886) or HBA-MRI (95.7% [88/92] vs. 90.6% [77/85], P=0.178).
Conclusions
The diagnostic value of EC in HCC diagnosis remains questionable, given its poor interobserver agreement and negligible impact on positive predictive values for HCC detection. This study challenges the emphasis on EC in certain diagnostic guidelines and suggests the need to re-evaluate its role in HCC imaging.

- Inter-reader Agreement for CT/MRI LI-RADS Category M Imaging Features: A Systematic Review and Meta-analysis
- Dong Hwan Kim, Sang Hyun Choi
- Received March 11, 2024 Accepted April 5, 2024 Published online April 15, 2024
- DOI: https://doi.org/10.17998/jlc.2024.04.05 [Accepted]

- 1,096 Views
- 36 Downloads
-
 Abstract
Abstract
 PDF
PDF - Backgrounds/Aims
To systematically evaluate inter-reader agreement in the assessment of individual Liver Imaging Reporting and Data System (LI-RADS) category M (LR-M) imaging features in computed tomography/magnetic resonance imaging (CT/MRI) LI-RADS v2018, and to explore the causes of poor agreement in LR-M assignment.
Methods
Original studies reporting inter-reader agreement for LR-M features on multiphasic CT or MRI were identified using the MEDLINE, EMBASE, and Cochrane databases. The pooled kappa coefficient (κ) was calculated using the DerSimonian–Laird random-effects model. Heterogeneity was assessed using Cochran’s Q test and I2 statistics. Subgroup meta-regression analyses were conducted to explore the study heterogeneity.
Results
In total, 24 eligible studies with 5,163 hepatic observations were included. The pooled κ values were 0.72 (95% confidence interval, 0.65–0.78) for rim arterial phase hyperenhancement, 0.52 (0.39–0.65) for peripheral washout, 0.60 (0.50–0.70) for delayed central enhancement, 0.68 (0.57–0.78) for targetoid restriction, 0.74 (0.65–0.83) for targetoid transitional phase/hepatobiliary phase appearance, 0.64 (0.49–0.78) for infiltrative appearance, 0.49 (0.30–0.68) for marked diffusion restriction, and 0.61 (0.48–0.73) for necrosis or severe ischemia. Substantial study heterogeneity was observed for all LR-M features (Cochran's Q test: p < 0.01; I2 ≥ 89.2%). Studies with a mean observation size of <3 cm, those performed using 1.5-T MRI, and those with multiple image readers, were significantly associated with poor agreement of LR-M features.
Conclusions
The agreement for peripheral washout and marked diffusion restriction was limited. The LI-RADS should focus on improving the agreement of LR-M features.

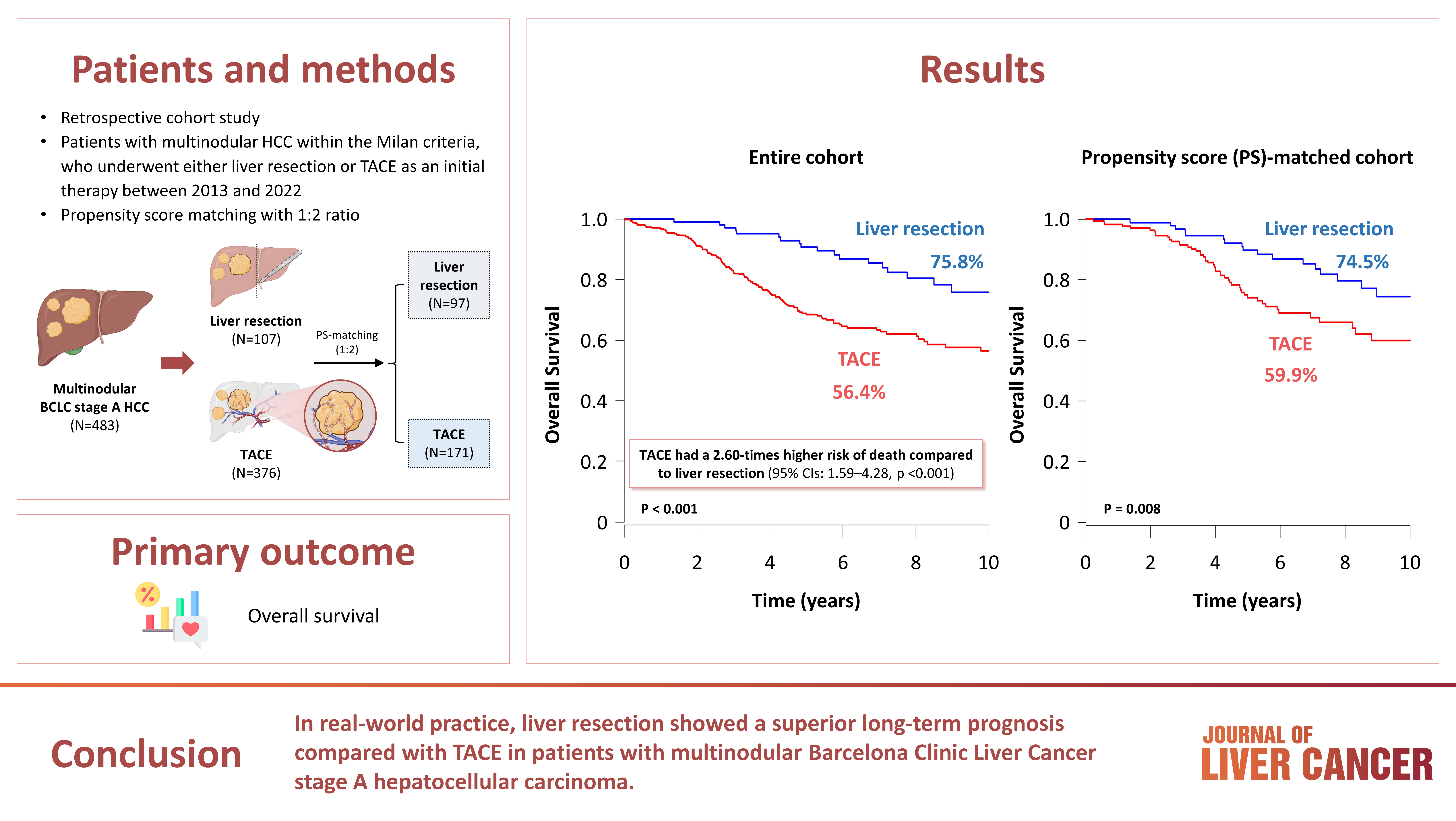
- Outcomes of Liver Resection and Transarterial Chemoembolization in Patients with Multinodular BCLC-A Hepatocellular Carcinoma
- Jiwon Yang, Won-Mook Choi, Danbi Lee, Ju Hyun Shim, Kang Mo Kim, Young-Suk Lim, Han Chu Lee, Deok-Bog Moon, Dong-Hwan Jung, Jonggi Choi
- Received March 3, 2024 Accepted March 25, 2024 Published online April 3, 2024
- DOI: https://doi.org/10.17998/jlc.2024.03.25 [Accepted]
- 760 Views
- 51 Downloads
-
 Abstract
Abstract
 PDF
PDF - Background
This study aimed to compare the outcomes of liver resection (LR) and transarterial chemoembolization (TACE) in patients with multinodular hepatocellular carcinoma (HCC) within the Milan criteria who were not eligible for liver transplantation.
Methods
We retrospectively analyzed 483 patients with multinodular HCC within the Milan criteria, who underwent either LR or TACE as an initial therapy between 2013 and 2022. The overall survival (OS) in the entire population and recurrence-free survival (RFS) in patients who underwent LR and TACE and achieved a complete response were analyzed. Propensity score (PS) matching analysis was also used for a fair comparison of outcomes between the two groups.
Results
Among the 483 patients, 107 (22.2%) and 376 (77.8%) underwent LR and TACE, respectively. The median size of the largest tumor was 2.0 cm, and 72.3% of the patients had two HCC lesions. The median OS and RFS were significantly longer in the LR group than in the TACE group (p <0.01 for both). In the multivariate analysis, TACE (adjusted hazard ratio [aHR], 1.81 and aHR, 2.41) and large tumor size (aHR, 1.43 and aHR, 1.44) were significantly associated with worse OS and RFS, respectively. The PS-matched analysis also demonstrated that the LR group had significantly longer OS and RFS than the TACE group (PS <0.05).
Conclusion
In this study, LR showed better OS and RFS than TACE in patients with multinodular Barcelona Clinic Liver Cancer stage A HCC. Therefore, LR can be considered an effective treatment option for these patients.


 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION
 First
First Prev
Prev



 Follow JLC on Twitter
Follow JLC on Twitter