Articles
- Page Path
- HOME > J Liver Cancer > Volume 24(1); 2024 > Article
-
Review Article
Changing etiology and epidemiology of hepatocellular carcinoma: Asia and worldwide -
Do Young Kim

-
Journal of Liver Cancer 2024;24(1):62-70.
DOI: https://doi.org/10.17998/jlc.2024.03.13
Published online: March 25, 2024
Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- Corresponding author: Do Young Kim, Department of Internal Medicine, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea E-mail: dyk1025@yuhs.ac
© 2024 The Korean Liver Cancer Association.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,792 Views
- 185 Downloads
- 2 Citations
- Abstract
- INTRODUCTION
- GEOGRAPHIC VARIATION OF HCC INCIDENCE AND MORTALITY
- CHANGING ETIOLOGY OF HCC IN ASIA AND WORLDWIDE
- GLOBAL BURDEN OF ALCOHOL-ASSOCIATED HCC
- EPIDEMIOLOGICAL CHARACTERISTICS OF NAFLD-ASSOCIATED HCC
- HCC INCIDENCE AMONG PATIENTS WITH NAFLD IN DIFFERENT REGIONS
- PROPORTION OF NAFLD AS AN ETIOLOGY OF HCC IN DIFFERENT REGIONS
- NAFLD AS AN EMERGING HCC ETIOLOGY IN ASIA
- HOW TO COPE WITH INCREASING PATIENTS WITH NAFLD-HCC
- Article information
- References
Abstract
- Approximately 80% of hepatocellular carcinoma (HCC) cases arise in sub-Saharan Africa and Eastern Asia, following a similarly high prevalence of chronic hepatitis B virus (HBV) carriers in these regions. The etiology and epidemiology of HCC have recently changed worldwide. Although HBV infection is the main contributor to HCC development, a slow but continuous decline in HBV infection rates has been reported since 1990. Owing to the widespread use of direct-acting antivirals, the incidence of hepatitis C virus-related HCC has remarkably decreased in Japan and European countries. In Korea, Taiwan, and Singapore, the incidence of HBV-related HCC has significantly decreased owing to vaccination against HBV. Globally, while HBV accounted for more than half of HCCs in 1990, this had decreased to 42% in 2019. In contrast, the proportion of patients with alcoholic- and nonalcoholic steatohepatitis (NASH) increased from 13% to 18% and from 5% to 6%, respectively. NASH-related HCC has characteristics that differ from those of virus-associated HCC. Compared with other etiologies, patients with NASHassociated HCC are older, have a higher body mass index, and have higher rates of type 2 diabetes mellitus, hypertension, hyperlipidemia, and cardiovascular disease. Nonalcoholic fatty liver disease (NAFLD)-associated HCC is also known to develop in the absence of cirrhosis, unlike alcohol-related and autoimmune liver diseases. Because patients with NAFLD usually have diabetes or obesity, surveying this population is challenging. Optimal selection of the target population and surveillance tools among patients with NAFLD needs to be determined.
- Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, accounting for 80-90% of all cases.1 This malignancy causes a serious health and economic burden globally, especially in Asia.2 It is the third leading cause of cancer-related death globally, with a 5-year survival rate of approximately 18%.3 After lung cancer, liver cancer (HCC and cholangiocarcinoma) ranked as the second most significant contributor to years of life lost due to cancer globally from 2005 to 2015, with a 4.6% increase in absolute years of life lost.4,5 Approximately 80% of HCC cases arise in sub-Saharan Africa and Eastern Asia, aligning with the high prevalence of chronic hepatitis B virus (HBV) carriers in these regions. HCC is the second leading cause of malignant deaths in Asia. The recent shift in overall trends in HCC incidence over time is explained by the worldwide vaccination program against HBV, active antiviral treatment using nucleoside/nucleotide analogs, introduction of direct-acting antiviral agents (DAA) for chronic hepatitis C, global efforts to eliminate viral hepatitis, and increasing rates of alcohol use and obesity, which influence the prevalence of alcoholic liver disease (ALD) and nonalcoholic fatty liver disease (NAFLD). The overall global burden of HCC has increased over time.
INTRODUCTION
- Significant differences exist worldwide in the incidence and mortality of HCC, primary due to differences in the timing and exposure to environmental and infectious risk factors, accessibility to healthcare resources, and the capability to detect early-stage HCC and provide potentially curative treatment.6 High incidence and mortality rates of 22-24 per 100,000 were reported for Thailand, Vietnam, and Cambodia in 2020, while Mongolia had the highest incidence and mortality rates worldwide at >80 per 100,000 persons. China had the highest number of individuals affected by HCC, followed by Japan, Thailand, and Vietnam (Table 1).7 The GLOBOCAN 2020 data demonstrates a trend that many countries with previously low HCC incidence, including Iran, Afghanistan, Qatar, Azerbaijan, Iraq and Nepal, have experienced a tremendous increase in the past 2 years.8 In the United States, the incidence of HCC has tripled over the last four decades, possibly due to the maturity of the patient pool with chronic hepatitis C. The burden of HCC is expected to reach 22 million cases in the next 2 decades in this country.9 The incidence in Japan has dropped drastically with a significant reduction in the population infected by hepatitis C virus (HCV). Similarly, HCC incidence in China is declining owing to increasing vaccination against hepatitis B.10 In Korea, the age-standardized incidence of liver cancer significantly decreased from 28.9 in 1999 to 19.7 in 2014 and 16.1 in 2019.11 The age-standardized death rate from liver cancer notably declined from 24.7 in 1999 to 16.4 in 2014 and further to 11.5 in 2020. However, the annual crude incidence rate of liver cancer has risen over the last 2 decades, from 28.1 in 1999 to its peak at 32.8 in 2010 and 31-32 in 2015. Additionally, the yearly absolute number of deaths has also increased over the same period, rising by 19.4% from 9,682 in 1999 to 11,566 in 2014, and then decreasing by 8.6% to 10,565 in 2020.11 Such a discrepancy between crude mortality rate and age-standardized mortality rate might be due to anti-HBV vaccination, widespread use of antiviral agents and the aging population.12 In 2019, the Western Pacific region demonstrated the highest frequency of incident liver cancer cases (n=295,484), deaths (n=254,054), and disability-adjusted life-years (DALYs; 6.7 million). However, interestingly, the US had the greatest increase between 2010 and 2019 in liver cancer incident cases (+41%), deaths (+42%), and DALYs (+36%).13 Lastly, despite a slowly decreasing trend in global age-standardized incidence rates of HCC since the late 1990s, the total number of HCC cases has been increasing owing to aging and population growth. In 2019, approximately 747,000 cases of HCC were reported worldwide, a 70% increase from 1990. While the global age-standardized incidence rates have been decreasing slowly since 2000, the rates have been increasing in countries with high sociodemographic indices since 1990. For example, the incidence of HCC in the United States has increased 2-3 fold over the past 3 decades owing to the high prevalence of HCV infection and NAFLD.6
GEOGRAPHIC VARIATION OF HCC INCIDENCE AND MORTALITY
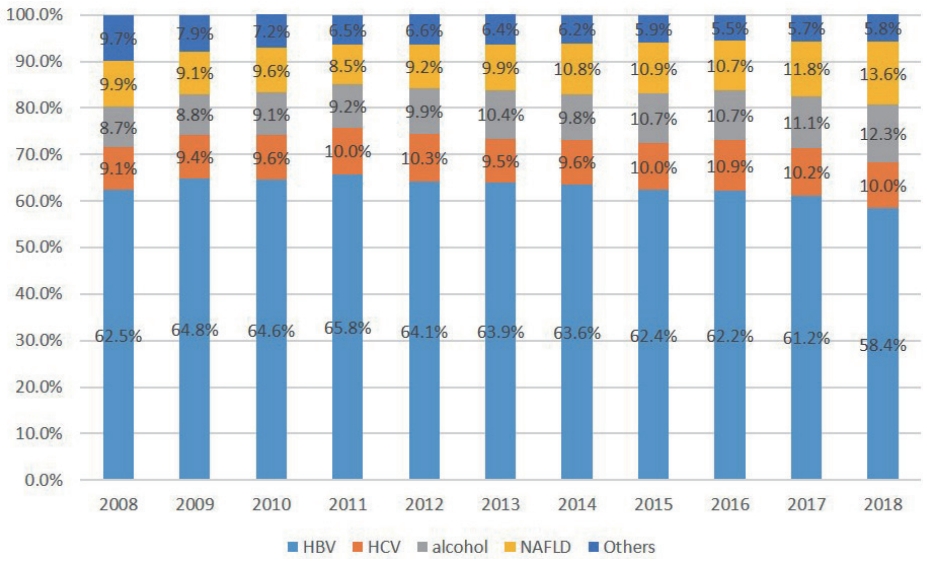
- Chronic HBV infection is a major global health problem with high prevalence, that can lead to cirrhosis as well as HCC.14,15 HBV is endemic in East and South-East Asia with the exception of Japan.16 Globally, an estimated 71 million individuals were infected with HCV in 2015.17 The annual risk of developing HCC with chronic hepatitis C ranges from 1.2% to 1.7%, and for individuals with cirrhosis, it varies from 1.4% to 2.5%.18 Although HBV is the primary cause of HCC, there has been a gradual but steady decrease in HBV infection rates in both global and Asia populations since 1999, according to the Global Burden of Diseases.19 Currently, the highest incidence and prevalence rates of HCC are observed in East Asia, North Africa, and Southeast Asia, regions where there is a higher prevalence of chronic HBV infection, contributing to over half of the global HCC cases. Conversely, cases associated with HCV, the main risk factor in the Mediterranean region, the United States, and Pakistan, have notably decreased due to the use of DAAs.20-22 Notably, in Taiwan, the mortality rate from HCC significantly dropped among individuals aged 5 to 29 years, from 0.81 deaths per 100,000 to 0.05 per 100,000, during 2001-2004, among those born in 1977-1980, attributed to the immunization campaign in 1984.23 Similarly, Hong Kong has witnessed a significant decline in HCC incidence across all age groups over the past 25 years, partly due to a decline in HBV infection rates since the introduction of universal HBV vaccination in 1988.24 According to the Global Burden of Disease study,25 there are temporal and geographical differences in HCC etiologies. Clearly, there was a transition from chronic viral hepatitis to a non-viral etiology between 1990 and 2019. Although HBV accounted for more than half of all cases in 1990, the proportion decreased to 42% in 2019. In contrast, the proportion of patients with alcoholic and non-alcoholic steatohepatitis (NASH) increased from 13% to 18% and from 5% to 6%, respectively. Regarding the distribution of HCC etiologies in various world regions in 2019, chronic viral hepatitis was still the main cause of HCC in Asia and most African regions, whereas non-viral causes such as NASH and ALD contributed to the majority of HCC cases in North America, Europe, and Australia (Fig. 1).25,26 In Korea, the hepatitis B surface antigen (HBsAg) positivity rates were high, exceeding 8% in the 1980s. However, with the introduction of HBV vaccination in 1983 and the implementation of the national immunization program in 1985, HBsAg-positive rates have decreased. These rates have further decreased since 2002, owing to the implementation of a perinatal infection prevention program. Fig. 2 shows the change in the proportion of HCV as an etiology of HCC between 2008 and 2018. The incidence of HBV-related HCC decreased, while the incidence of alcohol- and NAFLD-associated HCC increased from 8.7% and 9.9% to 12.3% and 13.6%, respectively (Fig. 2).27 Of note, HBV is still the most common etiology in Asia. In 1990, HBV infection caused more than half of all HCC cases worldwide. In 2019, this figure had decreased to 41%. Nonetheless, it remains the most common cause of HCC worldwide. In the Asia-Pacific region, HBV infection is the major cause of HCC, accounting for more than 60% of all HCC cases. In Korea, 70-80% of patients with HCC have current or past HBV infection.
CHANGING ETIOLOGY OF HCC IN ASIA AND WORLDWIDE
- ALD is one of the most prevalent liver diseases in the United States and Europe.28 Chronic, excessive alcohol intake, defined as consuming >40 g of pure alcohol per day (equivalent to 375 mL of 13 vol% wine or >1 L of 5 vol% beer) over an extend period, poses the greatest risk of ALD.29 However, a recent meta-analysis has demonstrated even consuming 12-24 g of alcohol per day chronically increases the risk of cirrhosis compared to abstaining from alcohol.29 When all World Health Organization regions are considered, adult per capita alcohol consumption has risen by approximately 10% over the past 25 years, mostly owing to significant increases in Asia (particularly in China and India) and Africa, whereas in North and South America and in Europe consumption decreased by 1% and 10%, repectively.30 In 2019, an estimated 19% of all liver cancer deaths worldwide were attributed to alcohol, lower than those caused by HBV infection (40%) and HCV infection (29%).13 Regional differences were observed in the percentage of liver cancer deaths related to alcohol, with Europe having the highest percentage (35%) and the eastern Mediterranean region the lowest (10%).13 Alcohol was the second-fastest rising contributor to the global liver cancer age-standardized death rate, with the Americas experiencing the highest increase. Global alcohol per capita consumption is projected to continue rising, especially in the Western Pacific and Southeast Asia, due to economic growth in densely populated regions.31 Therefore, more effective strategies must be put in place to reduce alcohol consumption.
GLOBAL BURDEN OF ALCOHOL-ASSOCIATED HCC
- NAFLD is the most common liver disease in the world. Its global prevalence in 2016 was approximately 25%, ranging from 13% in Africa to 42% in southeast Asia, with a projected 15-56% rise by 2030.32,33 In particular, the prevalence of NASH is projected to increase by up to 56% between 2016 and 2030 in China, France, Germany, Italy, Spain, Japan, the UK and the United States.33 While NAFLD, being related to the metabolic syndrome, has been considered prevalent primary in the Western world, recent years have seen its rise in Asia, paralleling lifestyle changes amid rapid economic growth. Studies reveal a rise in NAFLD prevalence in Asian countries from 25.3% (1999-2005) to 33.9% (2012-2017), underscoring this trend.34 Compared with other etiologies, patients with NASH-associated HCC are older (mean difference, 5.6 years); have higher body mass index (mean difference, 3 kg/m2); and have higher rates of type 2 diabetes mellitus (DM) (odds ratio [OR], 4.3), hypertension (OR, 2.8), hyperlipidemia (OR, 3.4), and cardiovascular disease (OR, 2.2).35 NAFLD-associated HCC is also well known to develop in the absence of cirrhosis, unlike liver diseases of other etiologies such as alcohol-related and autoimmune liver disease.36,37 In a Korean study, the clinical and survival outcomes of NAFLD-HCC and HBV-HCC were compared. The 232 patients underwent surgical resection; 32 had NAFLD-HCC and 200 had HBV-HCC. Before propensity score match (PSM), cirrhosis was more frequently detected in patients with HBV-HCC (55% vs. 15%, P<0.001) and the average tumor size was larger in the NAFLD-HCC group than in the HBV-HCC group (4.4±3.3 cm vs. 3.4±1.8 cm, P=0.014). After PSM, the 5-year overall survival rates were similar (60% vs. 63%, P=0.978) between the two groups.38 NAFLD has now become the fastest-growing cause of HCC among liver transplant recipients and candidates on the waiting list in the United States,39 with similar trends observed in Europe, South Korea, and Southeast Asia over the past 2 decades.40,41
EPIDEMIOLOGICAL CHARACTERISTICS OF NAFLD-ASSOCIATED HCC
- The incidence of HCC among patients with NAFLD differs substantially according to the stage of hepatic fibrosis and the presence of accompanying metabolic diseases such as obesity or DM. In the United States and Europe, the annual HCC incidence in cohorts of patients with NASH ranges from 0.7% to 2.6%.42,43 It was found, in a United States study, that 10 of 149 patients with NAFLD who had biopsy-proven cirrhosis developed HCC over 10 years.43 The HCC incidence in patients with cirrhosis due to NASH was higher in older patients, in males, and in patients with DM and higher alcohol consumption.44 The annual incidence of HCC was found to be 0.5% in a cohort of 41 patients with cirrhosis and NASH in a prospectively conducted study in India.45 Emerging evidence suggests that HCC can develop in NASH patients without cirrhosis; for instance an Italian study of 145 patients with NAFLD-associated HCC found that half of them lacked cirrhosis.46 It is noteworthy that patients without cirrhosis mainly had NASH with advanced fibrosis rather than simple fatty liver without fibrosis, emphasizing that the stage of fibrosis might be meaningful in hepatocarcinogenesis in the absence of cirrhosis.47 The incidence of HCC in patients without cirrhosis with NAFLD or NASH ranges from 0.1 to 1.3 per 1,000 patient-years.48 Though the HCC incidence among patients with NAFLD is significantly lower in those without cirrhosis than in those with cirrhosis, the HCC prevalence among patients with noncirrhotic NAFLD is high owing to the high frequency of patients who have NAFLD with fibrosis of any stage. A Japanese study reported an annual HCC incidence of 0.043%, based on a cohort of 6,508 patients with NAFLD diagnosed using ultrasonography. The cohort consisted largely of patients without cirrhosis or simple fatty liver because the aspartate aminotransferase to platelet ratio index was >1.5, in only 2.8% of patients.49 A Korean study of 8,721 patients with NAFLD diagnosed using ultrasonography demonstrated an HCC incidence of 23 per 100,000 patient-years. In the study, 25% of patients had an intermediate or high NAFLD fibrosis score.50 In a study of a Taiwanese cohort, the authors found a 10-year HCC incidence of 2.73%, which was similar to that observed in the Korean study.51
HCC INCIDENCE AMONG PATIENTS WITH NAFLD IN DIFFERENT REGIONS
- Globally, the estimated proportion of patients with HCC with NAFLD ranges from 1% to 38% in different countries/regions. In a study using a Markov model, it was suggested that the incidence of NAFLD-associated HCC in England would increase by 88% between 2016 and 2030, from 850 to 1,600 cases.33 Among European countries, Germany is projected to have the highest prevalence of NAFLD-associated HCC (4,090 cases) in 2030 because of its high projected incidence of NASH (4.7 million by 2030).33 The prevalence of cryptogenic HCC in Southeast Asia ranges from 12.6% in Singapore to 24.9% in the Philippines.52 In the United States, a moderate proportion of HCC is ascribed to NAFLD. In Brazil, the prevalence of NAFLD-related HCC is rapidly increasing. Among patients with HCC who require liver transplantation, those with NASH comprise the second largest group after those with HCV infection. The prevalence of NASH-associated HCC increased from 8.3% in 2002 to 13.5% in 2012 in a study using the United Network for Organ Sharing registry.53 The prevalence of NAFLD-related HCC increased from 2.6% (1995-1999) to 19.5% (2010-2014) in a French study with 323 patients with HCC who received liver resection at two centers between 1995 and 2014.54 In the most of patients with HCC in China, HBV is still the dominant etiology. Only 1% of 8,683 Chinese patients with HCC were ascribed to NAFLD in a study with patients who were diagnosed between 2005 and 2011.55 However, with increasing numbers of patients with NAFLD, estimated to be 243.7 million currently, and in the near future due to ageing population, the prevalence of NAFLD-related HCC is projected to rise from 14,090 cases in 2016 to 26,240 cases by 2030.33,56
PROPORTION OF NAFLD AS AN ETIOLOGY OF HCC IN DIFFERENT REGIONS
- Previously, NAFLD was predominantly considered a liver disease affecting Western populations. However, since 1990, the prevalence of NAFLD in Asia has risen significantly. In 2019, the Asian population accounted for 48.3% of global NAFLD-associated liver complications such as cirrhosis and HCC, surpassing even European and United States population.57 In China, both NASH and ALD contributed to approximately 13% of HCC cases in 2019,31 possibly driven by factors such as the rapid economic growth, increased alcohol consumption (from 4 L per capita in 2005 to 7 L in 2016), sedentary lifestyles, and escalating rates of obesity and DM (increasing by 2-3% between 2014 and 2018).58 By 2020, NASH and ALD affected 2-6% and 1-8% of the general population, respectively, with a projected 48% increase in NASH prevalence by 2030.59 In Japan, HCC prevalence has surged over the years, due to the increase in NASH and ALD.31 In particular, between 1996 and 2019, the prevalence of non-viral HCC escalated from 12% to 46%, mirroring the rise in obesity, metabolic syndrome, and alcoholism in this country.60 The prevalence rate of NAFLD in Japan nearly doubled from 13% (in the early 1990s) to 25-35% (in the early 2000s), and is predicted to affect 45% of the population by 2040.61 In Korea, a study revealed a rise in the proportion of HCC associated with NAFLD from 3.8% in 2001-2005 to 12.2% in 2006-2010.40 While data from other countries are lacking, the incidence of NAFLD-associated HCC is expected to surge in the future, given the development of NAFLD in many Asian countries over the past 2 decades due to sedentary lifestyles and over-nutrition.62
NAFLD AS AN EMERGING HCC ETIOLOGY IN ASIA
- To increase the number of patients with NAFLD globally, physicians need to control NAFLD epidemiology, screen for NAFLD among patients with metabolic diseases, identify surveillance methods and targets for patients with NAFLD, and optimize treatment for patients with NAFLD-associated HCC. The description of measures to prevent NAFLD in the general population is beyond the scope of this study. Type 2 DM is one of the strongest risk factors for the development of NASH, advanced fibrosis/cirrhosis, HCC, and mortality.63,64 It is estimated that 40-70% of patients with type 2 DM have underlying NAFLD, and among those, 37% have NASH and 17% will develop advanced fibrosis.65 NASH, and most importantly, the presence of advanced liver fibrosis, are key determinants of long-term prognosis and are associated with a high rate of liver-related mortality.66 Therefore, identifying patients at high risk earlier in the disease course is critical to prevent and monitor the risk of liver-related complications, such as liver failure and HCC. Although NAFLD is a prevalent disease in the general population, only a minority of patients with NAFLD develop NASH and advanced liver fibrosis, placing them at a higher risk of developing liver-related complications. This group of patients should be evaluated and managed by specialists to prevent and monitor liver-related complications. A recent publication by the United States members of the Global NASH Council recommended stratifying patients based on metabolic risk factors, including type 2 DM, utilizing fibrosis-4 (FIB-4) as the initial assessment tool. In a patients with DM, stratification would be by identifying one additional metabolic syndrome feature (weight circumference >40 inches in men, 35 inches in women; triglycerides ≥150 mg/dL; high density lipoprotein-cholesterol <40 mg/dL in men, 50 mg/dL in women; systolic blood pressure ≥130 mmHg and/or diastolic pressure ≥85 mmHg). For those without DM, at-risk patients are identified by having three metabolic risk factors (including elevated fasting glucose ≥100 mg/dL). Patients with a FIB-4 score ≥1.3 should undergo further evaluation by a liver specialist.67 The use of a noninvasive scoring system, such as NAFLD fibrosis score as well as FIB-4, is the simplest and most accurate strategy to identify patients at high risk of advanced fibrosis.68 In another recent article, the authors recommend the use of FIB-4 and/or vibration controlled transient elastography (VCTE) according to local resources, availability, and clinical context. The proposed algorithm involves a first-step annual FIB-4 score followed by VCTE for those with indeterminate or a high-risk score (FIB-4 ≥1.3). Patients at low risk (FIB-4 <1.3 or VCTE <8 kPa) can be followed up by primary care physicians for lifestyle changes and yearly calculation of FIB-4, while patients at high risk (FIB-4 ≥1.3 or VCTE ≥8 kPa) should be referred to liver-specialist clinics for further assessment and evaluation.69 As the risk of liver fibrosis increases according to the number of metabolic risk factors such as hypertension and hyperlipidemia, it might be important to pay more attention to the patients with multiple metabolic risk factors and to proactively monitoring NASH progression in these patients. Because nearly one-fourth of NAFLD-related HCC cases occur in the absence of cirrhosis, it is difficult to include patients with noncirrhotic NAFLD in the surveillance program. However, these patients have a very low annual HCC incidence of 0.008 per 100 person-years, so surveillance is not cost-effective in this population.70 The American Association for the Study of Liver Diseases guidelines recommend HCC surveillance for patients with cirrhotic NAFLD, but not for patients with noncirrhotic NAFLD.71 On the other hand, the European Association for the Study of the Liver (EASL) highlights the importance of categorizing those who should be screened for HCC, thus EASL guidelines state that patients with noncirrhotic stage 3 fibrosis should be included in the surveillance program, although the evidence level is low.72 The guidelines also raise the issue of optimal surveillance tool for patients with NAFLD/NASH. Obesity makes ultrasound screening more difficult in these patients. Because the use of computed tomography or magnetic resonance imaging is not cost-effective, the development of appropriate surveillance tools (serological or radiological) for this population is required.
- The etiology and epidemiology of HCC are rapidly changing worldwide. This trend indicates a transition from viral to non-viral causes and an increasing incidence of alcohol- and NAFLD-related HCC. Despite vaccination and treatment, HBV and HCV currently remain the most common etiologies in Asia and worldwide, while NASH is the fastest growing etiology of HCC in the world. To cope with this, effective cooperation between the government and academic society is necessary.
HOW TO COPE WITH INCREASING PATIENTS WITH NAFLD-HCC
-
Conflict of Interest
The author has no conflicts of interest to disclose.
-
Ethics Statement
This review article is fully based on articles which have already been published and did not involve additional patient participants. Therefore, IRB approval is not necessary.
-
Funding Statement
None.
-
Data Availability
Not applicable.
-
Author Contribution
Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing - original draft, writing - review & editing: DYK
Article information

- 1. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. ArticlePubMedPDF
- 2. Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology 2019;156:477−491.e1.ArticlePubMedPMC
- 3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7−33.ArticlePubMedPDF
- 4. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264−1273. e1.ArticlePubMedPMC
- 5. Tang A, Hallouch O, Chernyak V, Kamaya A, Sirlin CB. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radiol (NY) 2018;43:13−25.ArticlePubMedPDF
- 6. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589−604.ArticlePubMedPMCPDF
- 7. Tran NH. Shifting epidemiology of hepatocellular carcinoma in Far Eastern and Southeast Asian patients: explanations and implications. Curr Oncol Rep 2022;24:187−193.ArticlePubMedPDF
- 8. Zhang CH, Cheng Y, Zhang S, Fan J, Gao Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int 2022;42:2029−2041.ArticlePubMedPDF
- 9. Stewart BW, Wild CP. World cancer report 2014 [Internet]. Lyon: International Agency for Research on Cancer; [cited 2019 Jul 27]. Available from: https://publications.iarc.fr/Non-Series-Publications/WorldCancer-Reports/World-Cancer-Report-2014
- 10. Kao JH, Chen DS. Changing disease burden of hepatocellular carcinoma in the Far East and Southeast Asia. Liver Int 2005;25:696−703.ArticlePubMed
- 11. Korean Central Cancer Registry. Annual report of Korean central cancer registry 2019 [Internet]. Goyang: Korea Central Cancer Registry; [cited 2024 Jan 18]. Available from: https://ncc.re.kr/cancerStatsView.ncc?bbsnum=598&searchKey=total&searchValue=&pageNum=1
- 12. Korean Liver Cancer Association (KLCA), National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. J Liver Cancer 2023;23:1−120.ArticlePubMedPMCPDF
- 13. Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab 2022;34:969−977.e2.ArticlePubMedPMC
- 14. Chan HL, Jia J. Chronic hepatitis B in Asia-new insights from the past decade. J Gastroenterol Hepatol 2011;26 Suppl 1:131−137.ArticlePubMed
- 15. Chan SL, Wong VW, Qin S, Chan HL. Infection and cancer: the case of hepatitis B. J Clin Oncol 2016;34:83−90.ArticlePubMed
- 16. Umemura T, Ichijo T, Yoshizawa K, Tanaka E, Kiyosawa K. Epidemiology of hepatocellular carcinoma in Japan. J Gastroenterol 2009;44 Suppl 19:102−107.ArticlePubMedPDF
- 17. World Health Organization (WHO). Global hepatitis report, 2017 [Internet]. Geneva: WHO; [cited 2021 Apr 17]. Available from: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/
- 18. Mahale P, Torres HA, Kramer JR, Hwang LY, Li R, Brown EL, et al. Hepatitis C virus infection and the risk of cancer among elderly US adults: a registry-based case-control study. Cancer 2017;123:1202−1211.ArticlePubMedPMCPDF
- 19. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204−1222.PubMedPMC
- 20. Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol 2022;77:1598−1606.ArticlePubMedPMC
- 21. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 2017;153:996−1005.e1.ArticlePubMed
- 22. Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol 2010;53:348−356.ArticlePubMed
- 23. Chiang CJ, Yang YW, You SL, Lai MS, Chen CJ. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. JAMA 2013;310:974−976.ArticlePubMed
- 24. Liu Y, Liu L. Changes in the epidemiology of hepatocellular carcinoma in Asia. Cancers (Basel) 2022;14:4473. ArticlePubMedPMC
- 25. Yang J, Pan G, Guan L, Liu Z, Wu Y, Liu Z, et al. The burden of primary liver cancer caused by specific etiologies from 1990 to 2019 at the global, regional, and national levels. Cancer Med 2022;11:1357−1370.ArticlePubMedPMCPDF
- 26. Toh MR, Wong EYT, Wong SH, Ng AWT, Loo LH, Chow PK, et al. Global epidemiology and genetics of hepatocellular carcinoma. Gastroenterology 2023;164:766−782.ArticlePubMed
- 27. Korean Association for the Study of the Liver. 2021 hepatocellular carcinoma factsheet in Korea [Internet]. Seoul: Korean Association for the study of the Liver; [cited 2024 Feb 2]. Available from: https://new.kasl.org/bbs/index.html?code=factsheet&category=&gubun=&idx=&page=1&number=4644&mode=view&order=&sort=&keyfield=&key=
- 28. Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol 2013;59:160−168.ArticlePubMed
- 29. Rehm J, Taylor B, Mohapatra S, Irving H, Baliunas D, Patra J, et al. Alcohol as a risk factor for liver cirrhosis: a systematic review and metaanalysis. Drug Alcohol Rev 2010;29:437−445.ArticlePubMed
- 30. Shield KD, Rylett M, Rehm J. Public health successes and missed opportunities: trends in alcohol consumption and attributable mortality in the WHO European Region, 1990-2014 [Internet]. Geneva: World Health Organization; [cited 2024 Feb 2]. Available from: https://iris.who.int/handle/10665/329489
- 31. Huang DQ, Mathurin P, Cortez-Pinto H, Loomba R. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol 2023;20:37−49.ArticlePubMedPMCPDF
- 32. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73−84.ArticlePubMed
- 33. Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol 2018;69:896−904.ArticlePubMed
- 34. Farrell GC, Wong VW, Chitturi S. NAFLD in Asia--as common and important as in the West. Nat Rev Gastroenterol Hepatol 2013;10:307−318.ArticlePubMedPDF
- 35. Tan DJH, Ng CH, Lin SY, Pan XH, Tay P, Lim WH, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol 2022;23:521−530.ArticlePubMedPMC
- 36. Stine JG, Wentworth BJ, Zimmet A, Rinella ME, Loomba R, Caldwell SH, et al. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther 2018;48:696−703.ArticlePubMedPMCPDF
- 37. Desai A, Sandhu S, Lai JP, Sandhu DS. Hepatocellular carcinoma in noncirrhotic liver: a comprehensive review. World J Hepatol 2019;11:1−18.ArticlePubMedPMC
- 38. Jung YB, Yoo JE, Han DH, Kim KS, Choi JS, Kim DY, et al. Clinical and survival outcomes after hepatectomy in patients with non-alcoholic fatty liver and hepatitis B-related hepatocellular carcinoma. HPB (Oxford) 2021;23:1113−1122.ArticlePubMed
- 39. Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol 2019;17:748−755.e3.ArticlePubMed
- 40. Cho EJ, Kwack MS, Jang ES, You SJ, Lee JH, Kim YJ, et al. Relative etiological role of prior hepatitis B virus infection and nonalcoholic fatty liver disease in the development of non-B non-C hepatocellular carcinoma in a hepatitis B-endemic area. Digestion 2011;84 Suppl 1:17−22.ArticlePubMedPDF
- 41. Liew ZH, Goh GB, Hao Y, Chang PE, Tan CK. Comparison of hepatocellular carcinoma in patients with cryptogenic versus hepatitis B etiology: a study of 1079 cases over 3 decades. Dig Dis Sci 2019;64:585−590.ArticlePubMedPDF
- 42. Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972−1978.ArticlePubMed
- 43. Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 2006;43:682−689.ArticlePubMed
- 44. Yang JD, Ahmed F, Mara KC, Addissie BD, Allen AM, Gores GJ, et al. Diabetes is associated with increased risk of hepatocellular carcinoma in patients with cirrhosis from nonalcoholic fatty liver disease. Hepatology 2020;71:907−916.ArticlePubMedPMCPDF
- 45. Amarapurkar DN, Dharod M, Gautam S, Patel N. Risk of development of hepatocellular carcinoma in patients with NASH-related cirrhosis. Trop Gastroenterol 2013;34:159−163.ArticlePubMed
- 46. Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology 2016;63:827−838.ArticlePubMedPDF
- 47. Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021;18:223−238.ArticlePubMedPMCPDF
- 48. Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, et al. Risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Gastroenterology 2018;155:1828−1837.e2.ArticlePubMedPMC
- 49. Kawamura Y, Arase Y, Ikeda K, Seko Y, Imai N, Hosaka T, et al. Largescale long-term follow-up study of Japanese patients with non-alcoholic fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol 2012;107:253−261.ArticlePubMedPDF
- 50. Kim GA, Lee HC, Choe J, Kim MJ, Lee MJ, Chang HS, et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol 2017;68:140−146.Article
- 51. Lee TY, Wu JC, Yu SH, Lin JT, Wu MS, Wu CY. The occurrence of hepatocellular carcinoma in different risk stratifications of clinically noncirrhotic nonalcoholic fatty liver disease. Int J Cancer 2017;141:1307−1314.ArticlePubMedPDF
- 52. Goh KL, Razlan H, Hartono JL, Qua CS, Yoong BK, Koh PS, et al. Liver cancer in Malaysia: epidemiology and clinical presentation in a multiracial Asian population. J Dig Dis 2015;16:152−158.PubMed
- 53. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014;59:2188−2195.ArticlePubMed
- 54. Pais R, Fartoux L, Goumard C, Scatton O, Wendum D, Rosmorduc O, et al. Temporal trends, clinical patterns and outcomes of NAFLD-related HCC in patients undergoing liver resection over a 20-year period. Aliment Pharmacol Ther 2017;46:856−863.ArticlePubMedPDF
- 55. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int 2015;35:2155−2166.PubMedPMC
- 56. Zhou F, Zhou J, Wang W, Zhang XJ, Ji YX, Zhang P, et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: a systematic review and meta-analysis. Hepatology 2019;70:1119−1133.ArticlePubMedPDF
- 57. Golabi P, Paik JM, AlQahtani S, Younossi Y, Tuncer G, Younossi ZM. Burden of non-alcoholic fatty liver disease in Asia, the Middle East and North Africa: data from Global Burden of Disease 2009-2019. J Hepatol 2021;75:795−809.ArticlePubMed
- 58. Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, et al. Prevalence and treatment of diabetes in China, 2013-2018. JAMA 2021;326:2498−2506.ArticlePubMedPMC
- 59. Wang H, Gao P, Chen W, Yuan Q, Lv M, Bai S, et al. A cross-sectional study of alcohol consumption and alcoholic liver disease in Beijing: based on 74,998 community residents. BMC Public Health 2022;22:723. ArticlePubMedPMCPDF
- 60. Nakano M, Yatsuhashi H, Bekki S, Takami Y, Tanaka Y, Yoshimaru Y, et al. Trends in hepatocellular carcinoma incident cases in Japan between 1996 and 2019. Sci Rep 2022;12:1517. ArticlePubMedPMCPDF
- 61. Ito T, Ishigami M, Zou B, Tanaka T, Takahashi H, Kurosaki M, et al. The epidemiology of NAFLD and lean NAFLD in Japan: a meta-analysis with individual and forecasting analysis, 1995-2040. Hepatol Int 2021;15:366−379.ArticlePubMedPDF
- 62. Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol 2017;67:862−873.ArticlePubMed
- 63. Hossain N, Afendy A, Stepanova M, Nader F, Srishord M, Rafiq N, et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1224−1229.e2.ArticlePubMed
- 64. Stepanova M, Rafiq N, Makhlouf H, Agrawal R, Kaur I, Younoszai Z, et al. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD). Dig Dis Sci 2013;58:3017−3023.ArticlePubMedPDF
- 65. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol 2019;71:793−801.ArticlePubMed
- 66. Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology 2020;158:1611−1625.e12.ArticlePubMed
- 67. Younossi ZM, Corey KE, Alkhouri N, Noureddin M, Jacobson I, Lam B, et al. Clinical assessment for high-risk patients with non-alcoholic fatty liver disease in primary care and diabetology practices. Aliment Pharmacol Ther 2020;52:513−526.ArticlePubMedPDF
- 68. Armstrong MJ, Marchesini G. Referral pathways for NAFLD fibrosis in primary care - no longer a 'needle in a haystack'. J Hepatol 2019;71:246−248.ArticlePubMed
- 69. Barbosa JV, Lai M. Nonalcoholic fatty liver disease screening in type 2 diabetes mellitus patients in the primary care setting. Hepatol Commun 2020;5:158−167.ArticlePubMedPMCPDF
- 70. Orci LA, Sanduzzi-Zamparelli M, Caballol B, Sapena V, Colucci N, Torres F, et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Clin Gastroenterol Hepatol 2022;20:283−292.e10.ArticlePubMed
- 71. Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, et al. AASLD Practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023;78:1922−1965.ArticlePubMed
- 72. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182−236.ArticlePubMed
References
Figure & Data
References
Citations

- Immunotherapy as a Complement to Surgical Management of Hepatocellular Carcinoma
Susan J. Kim, Kaelyn C. Cummins, Allan Tsung
Cancers.2024; 16(10): 1852. CrossRef - Inflammatory Response in the Pathogenesis and Treatment of Hepatocellular Carcinoma: A Double-Edged Weapon
Linda Galasso, Lucia Cerrito, Valeria Maccauro, Fabrizio Termite, Irene Mignini, Giorgio Esposto, Raffaele Borriello, Maria Elena Ainora, Antonio Gasbarrini, Maria Assunta Zocco
International Journal of Molecular Sciences.2024; 25(13): 7191. CrossRef
 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation
- Download Citation
- Close
- Related articles
-
- Sonazoid contrast-enhanced ultrasonography for the diagnosis of hepatocellular carcinoma: strengths and shortcomings
- Imaging prognostication and tumor biology in hepatocellular carcinoma
- The efficacy of treatment for hepatocellular carcinoma in elderly patients
- Radiologic features of hepatocellular carcinoma related to prognosis
- Liquid biopsy for early detection and therapeutic monitoring of hepatocellular carcinoma

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION


 Follow JLC on Twitter
Follow JLC on Twitter