Search
- Page Path
- HOME > Search
Original Articles
- Downstaging with Atezolizumab-Bevacizumab: A Case Series
- Anand V. Kulkarni, Kumaraswamy P, Balachandran Menon, Anuradha Sekaran, Anuhya Rambhatl, Sowmya Iyengar, Manasa Alla, Shantan Venishetty, Sumana Kolar Ramachandra, Premkumar G V, Mithun Sharma, P. N Rao, Duvvur Nageshwar Reddy, Amit G. Singal
- Received April 3, 2024 Accepted May 12, 2024 Published online May 27, 2024
- DOI: https://doi.org/10.17998/jlc.2024.05.12 [Accepted]

- 1,047 Views
- 140 Downloads
-
 Abstract
Abstract
 PDF
PDF - Background/aims
Hepatocellular carcinoma (HCC) is generally diagnosed at an advanced stage, which limits curative treatment options for these patients. Locoregional therapy (LRT) is the standard approach to bridge and downstage unresectable HCC (uHCC) for liver transplantation (LT). Atezolizumab-bevacizumab (atezo-bev) can induce objective responses in nearly one-third of patients; however, the role and outcomes of downstaging using atezobev remains unknown.
Methods
In this retrospective single-center study, we included consecutive patients between November 2020 and August 2023, who received atezo-bev with or without LRT and were subsequently considered for resection/LT after downstaging.
Results
Of the 115 patients who received atezo-bev, 12 patients (10.4%) achieved complete or partial response and were willing to undergo LT; they (age: 58.5 years; women-17%; Barcelona Clinic Liver Cancer Stage System B/C:5/7) had received 3–12 cycles of atezo-bev, and 4 of them had received prior LRT. Three patients died before LT, while three were awaiting LT. Six patients underwent curative therapies: four underwent living donor LT after a median of 79.5 (54–114) days following the last atezo-bev dose, one underwent deceased donor LT 38 days after the last dose, and one underwent resection. All but one patient had complete pathologic response with no viable HCC. Three patients experienced wound healing complications, and one required re-exploration and succumbed to sepsis. After a median follow-up of 10 (4–30) months, none of the alive patients developed HCC recurrence or graft rejection.
Conclusions
Surgical therapy, including LT, is possible after atezo-bev therapy in wellselected patients after downstaging.

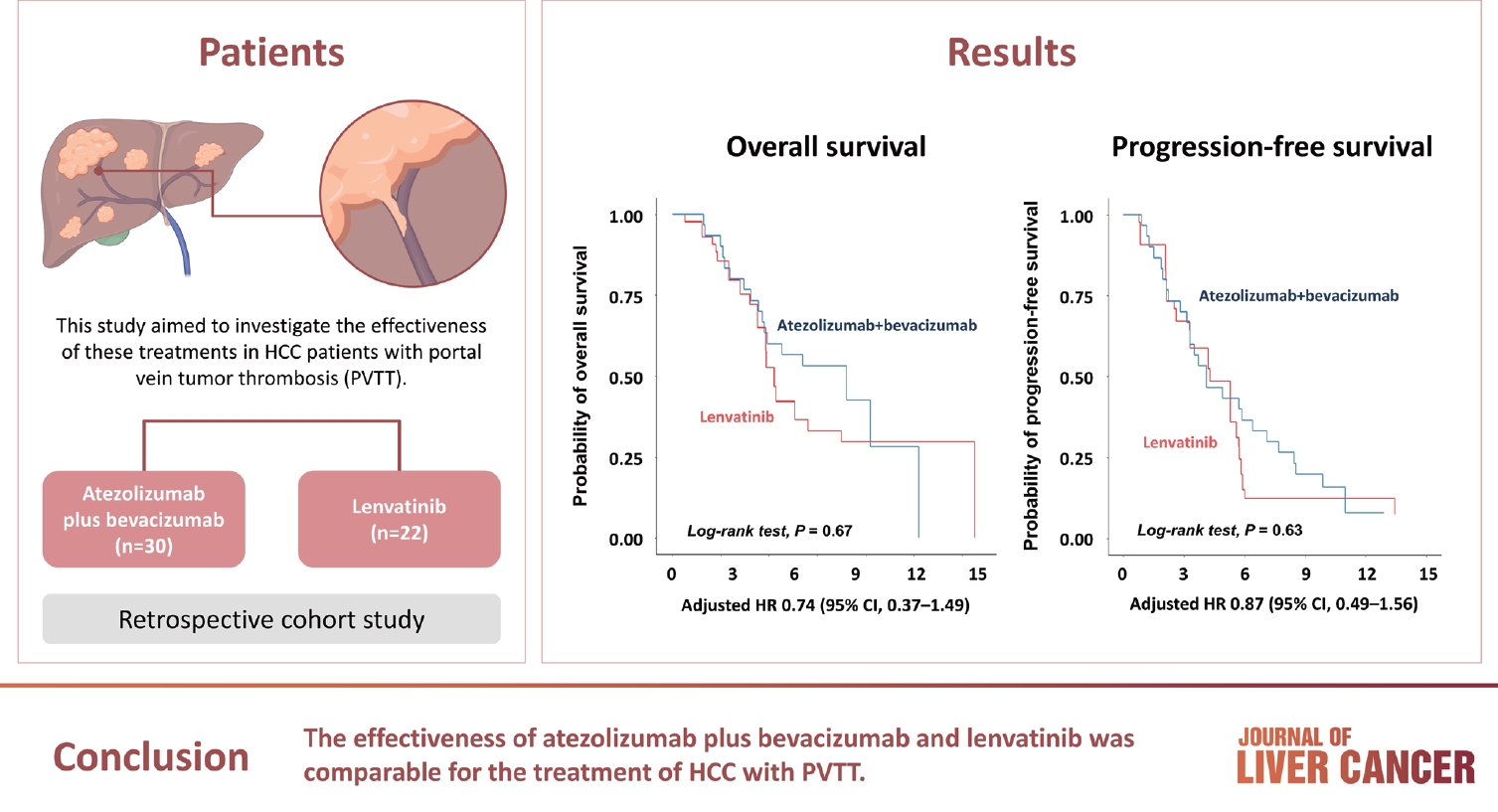
- Comparison of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma with portal vein tumor thrombosis
- Jeayeon Park, Yun Bin Lee, Yunmi Ko, Youngsu Park, Hyunjae Shin, Moon Haeng Hur, Min Kyung Park, Dae-Won Lee, Eun Ju Cho, Kyung-Hun Lee, Jeong-Hoon Lee, Su Jong Yu, Tae-Yong Kim, Yoon Jun Kim, Tae-You Kim, Jung-Hwan Yoon
- J Liver Cancer. 2024;24(1):81-91. Published online January 19, 2024
- DOI: https://doi.org/10.17998/jlc.2023.12.25
- 2,107 Views
- 167 Downloads
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background/Aim
Atezolizumab plus bevacizumab and lenvatinib are currently available as first-line therapy for the treatment of unresectable hepatocellular carcinoma (HCC). However, comparative efficacy studies are still limited. This study aimed to investigate the effectiveness of these treatments in HCC patients with portal vein tumor thrombosis (PVTT).
Methods
We retrospectively included patients who received either atezolizumab plus bevacizumab or lenvatinib as first-line systemic therapy for HCC with PVTT. Primary endpoint was overall survival (OS), and secondary endpoints included progressionfree survival (PFS) and disease control rate (DCR) determined by response evaluation criteria in solid tumors, version 1.1.
Results
A total of 52 patients were included: 30 received atezolizumab plus bevacizumab and 22 received lenvatinib. The median follow-up duration was 6.4 months (interquartile range, 3.9-9.8). The median OS was 10.8 months (95% confidence interval [CI], 5.7 to not estimated) with atezolizumab plus bevacizumab and 5.8 months (95% CI, 4.8 to not estimated) with lenvatinib (P=0.26 by log-rank test). There was no statistically significant difference in OS (adjusted hazard ratio [aHR], 0.71; 95% CI, 0.34-1.49; P=0.37). The median PFS was similar (P=0.63 by log-rank test), with 4.1 months (95% CI, 3.3-7.7) for atezolizumab plus bevacizumab and 4.3 months (95% CI, 2.6-5.8) for lenvatinib (aHR, 0.93; 95% CI, 0.51-1.69; P=0.80). HRs were similar after inverse probability treatment weighting. The DCRs were 23.3% and 18.2% in patients receiving atezolizumab plus bevacizumab and lenvatinib, respectively (P=0.74).
Conclusion
The effectiveness of atezolizumab plus bevacizumab and lenvatinib was comparable for the treatment of HCC with PVTT.

Review Articles
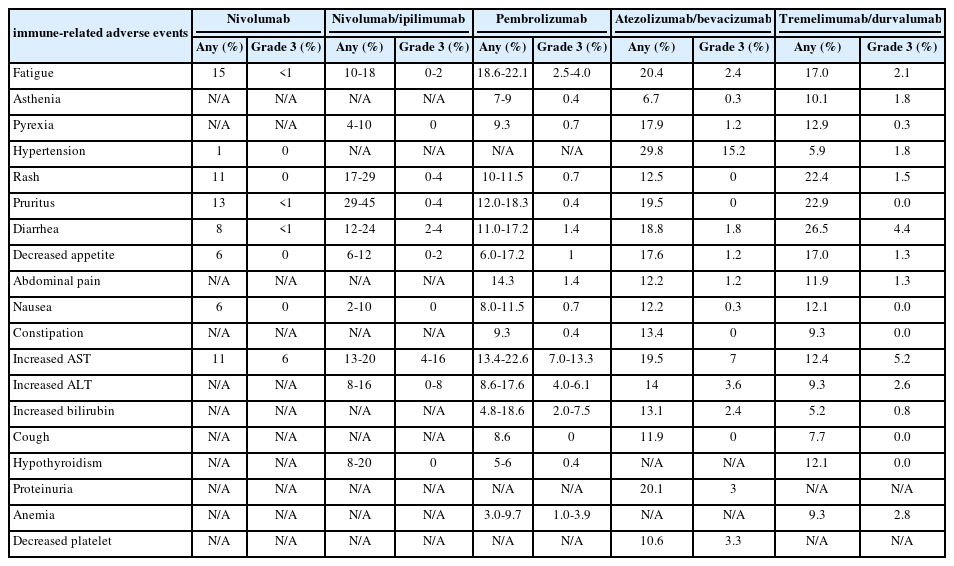
- Complications of immunotherapy in advanced hepatocellular carcinoma
- Young-Gi Song, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
- J Liver Cancer. 2024;24(1):9-16. Published online November 29, 2023
- DOI: https://doi.org/10.17998/jlc.2023.11.21
- 2,115 Views
- 122 Downloads
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF - Immune checkpoint inhibitors (ICIs) are highly effective in cancer treatment. However, the risks associated with the treatment must be carefully balanced against the therapeutic benefits. Immune-related adverse events (irAEs) are generally unpredictable and may persist over an extended period. In this review, we analyzed common irAEs reported in highly cited original articles and systematic reviews. The prevalent adverse reactions include fatigue, pyrexia, rash, pruritus, diarrhea, decreased appetite, nausea, abdominal pain, constipation, hepatitis, and hypothyroidism. Therefore, it is crucial to conduct evaluations not only of gastrointestinal organs but also of cardiac, neurologic, endocrine (including the frequently affected thyroid), and ophthalmic systems before commencing ICIs. This review further explores commonly reported types of irAEs, specific irAEs associated with each ICI agent, rare yet potentially fatal irAEs, and available treatment options for managing them.
-
Citations
Citations to this article as recorded by- Intrahepatic IgA complex induces polarization of cancer-associated fibroblasts to matrix phenotypes in the tumor microenvironment of HCC
Jong Geun Park, Pu Reun Roh, Min Woo Kang, Sung Woo Cho, Suhyun Hwangbo, Hae Deok Jung, Hyun Uk Kim, Ji Hoon Kim, Jae-Sung Yoo, Ji Won Han, Jeong Won Jang, Jong Young Choi, Seung Kew Yoon, Young Kyoung You, Ho Joong Choi, Jae Yong Ryu, Pil Soo Sung
Hepatology.2024;[Epub] CrossRef - Risk of Bleeding in Hepatocellular Carcinoma Patients Treated with Atezolizumab/Bevacizumab: A Systematic Review and Meta-Analysis
Young-Gi Song, Kyeong-Min Yeom, Eun Ae Jung, Sang Gyune Kim, Young Seok Kim, Jeong-Ju Yoo
Liver Cancer.2024; : 1. CrossRef
- Intrahepatic IgA complex induces polarization of cancer-associated fibroblasts to matrix phenotypes in the tumor microenvironment of HCC

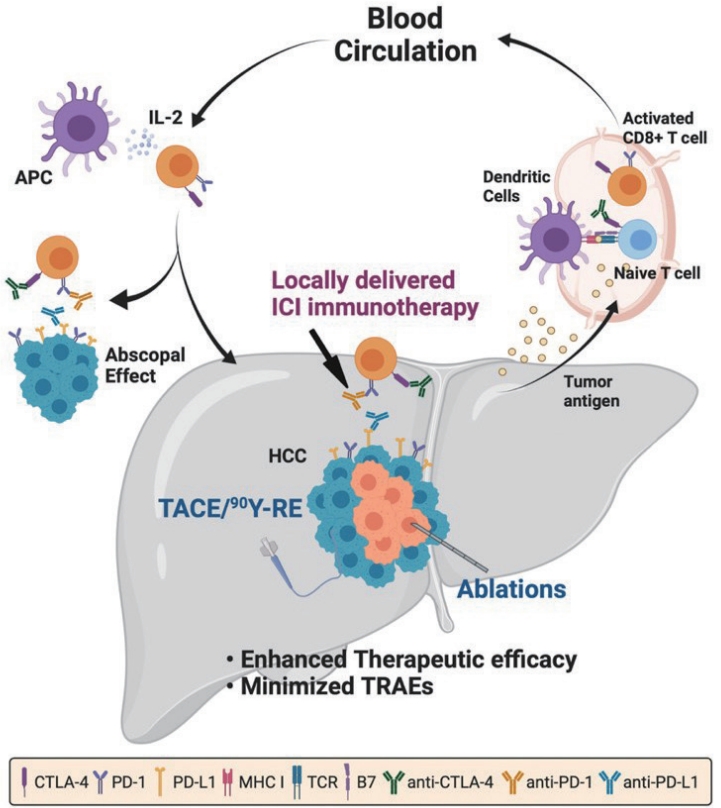
- Combination of interventional oncology local therapies and immunotherapy for the treatment of hepatocellular carcinoma
- Dong-Hyun Kim
- J Liver Cancer. 2022;22(2):93-102. Published online April 22, 2022
- DOI: https://doi.org/10.17998/jlc.2022.03.28
- 6,088 Views
- 181 Downloads
- 7 Citations
-
 Abstract
Abstract
 PDF
PDF - Interventional oncology (IO) local therapies of hepatocellular carcinoma (HCC) can activate anti-cancer immunity and it is potentially leading to an anti-cancer immunity throughout the body. For the development of an effective HCC treatment regime, great emphasis has been dedicated to different IO local therapy mediated immune modulation and possible combinations with immune checkpoint inhibitor immunotherapy. In this review paper, we summarize the status of combination of IO local therapy and immunotherapy, as well as the prospective role of therapeutic carriers and locally administered immunotherapy in advanced HCC.
-
Citations
Citations to this article as recorded by- Reduced-Dose or Discontinuation of Bevacizumab Might Be Considered after Variceal Bleeding in Patients with Hepatocellular Carcinoma Receiving Atezolizumab/Bevacizumab: Case Reports
Kyeong-Min Yeom, Young-Gi Song, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
Medicina.2024; 60(1): 157. CrossRef - CT-guided high dose rate brachytherapy can induce multiple systemic proteins of proliferation and angiogenesis predicting outcome in HCC
Lukas Salvermoser, Shraga Nahum Goldberg, Marianna Alunni-Fabbroni, Philipp Maximilian Kazmierczak, Moritz Nikolaus Gröper, Jan Niklas Schäfer, Elif Öcal, Tanja Burkard, Stefanie Corradini, Najib Ben Khaled, Agnese Petrera, Moritz Wildgruber, Jens Ricke,
Translational Oncology.2024; 43: 101919. CrossRef - Complications of immunotherapy in advanced hepatocellular carcinoma
Young-Gi Song, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
Journal of Liver Cancer.2024; 24(1): 9. CrossRef - Syngeneic N1-S1 Orthotopic Hepatocellular Carcinoma in Sprague Dawley Rat for the Development of Interventional Oncology-Based Immunotherapy: Survival Assay and Tumor Immune Microenvironment
Bongseo Choi, Jason Pe, Bo Yu, Dong-Hyun Kim
Cancers.2023; 15(3): 913. CrossRef - Preclinical Development and Validation of Translational Temperature Sensitive Iodized Oil Emulsion Mediated Transcatheter Arterial Chemo‐Immuno‐Embolization for the Treatment of Hepatocellular Carcinoma
Heegon Kim, Bongseo Choi, Samdeep K. Mouli, Hyunjun Choi, Kathleen R. Harris, Laura M. Kulik, Robert J. Lewandowski, Dong‐Hyun Kim
Advanced Healthcare Materials.2023;[Epub] CrossRef - The Current Evidence of Intensity-Modulated Radiotherapy for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
Won Il Jang, Sunmi Jo, Ji Eun Moon, Sun Hyun Bae, Hee Chul Park
Cancers.2023; 15(20): 4914. CrossRef - Inducing the Abscopal Effect in Liver Cancer Treatment: The Impact of Microwave Ablation Power Levels and PD-1 Antibody Therapy
Changli Liao, Guiyuan Zhang, Ruotong Huang, Linyuan Zeng, Bin Chen, Haitao Dai, Keyu Tang, Run Lin, Yonghui Huang
Pharmaceuticals.2023; 16(12): 1672. CrossRef
- Reduced-Dose or Discontinuation of Bevacizumab Might Be Considered after Variceal Bleeding in Patients with Hepatocellular Carcinoma Receiving Atezolizumab/Bevacizumab: Case Reports

- Advances in immune checkpoint inhibitors for hepatocellular carcinoma
- Ji Won Han, Su-Hyung Park
- J Liver Cancer. 2021;21(2):139-145. Published online September 30, 2021
- DOI: https://doi.org/10.17998/jlc.2021.09.24
- 3,975 Views
- 105 Downloads
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF - Hepatocellular carcinoma (HCC) is the fifth most common cancer, and the second leading cause of cancer-related death worldwide. Although recent advances in immune checkpoint inhibitor-based immunotherapy have initiated a new era for advanced HCC treatment, the majority of HCC patients receiving immune checkpoint blockades do not derive clinical benefit. Thus, there remains an urgent need for novel immunotherapeutic strategies with improved therapeutic efficacy. Here we review recent studies of immune checkpoint blockade in HCC, providing the necessary basis for the rational design of immunotherapy.
-
Citations
Citations to this article as recorded by- Systematic Review of Molecular Targeted Therapies for Adult-Type Diffuse Glioma: An Analysis of Clinical and Laboratory Studies
Logan Muzyka, Nicolas K. Goff, Nikita Choudhary, Michael T. Koltz
International Journal of Molecular Sciences.2023; 24(13): 10456. CrossRef - Integrative analysis of lactylation-related genes and establishment of a novel prognostic signature for hepatocellular carcinoma
Diankui Cai, Xiaoqing Yuan, D. Q. Cai, Ang Li, Sijia Yang, Weibang Yang, Jinxin Duan, Wenfeng Zhuo, Jun Min, Li Peng, Jinxing Wei
Journal of Cancer Research and Clinical Oncology.2023; 149(13): 11517. CrossRef - Editorial on Immune Checkpoint Inhibitors in the Treatment of Hepatocellular Carcinoma
Samantha M Ruff, Timothy M Pawlik
Immunotherapy.2023; 15(16): 1323. CrossRef
- Systematic Review of Molecular Targeted Therapies for Adult-Type Diffuse Glioma: An Analysis of Clinical and Laboratory Studies

- Systemic therapy for advanced hepatocellular carcinoma: consideration for selecting second-line treatment
- Bo Hyun Kim, Joong-Won Park
- J Liver Cancer. 2021;21(2):124-138. Published online September 30, 2021
- DOI: https://doi.org/10.17998/jlc.2021.09.23
- 4,422 Views
- 120 Downloads
- 2 Citations
-
 Abstract
Abstract
 PDF
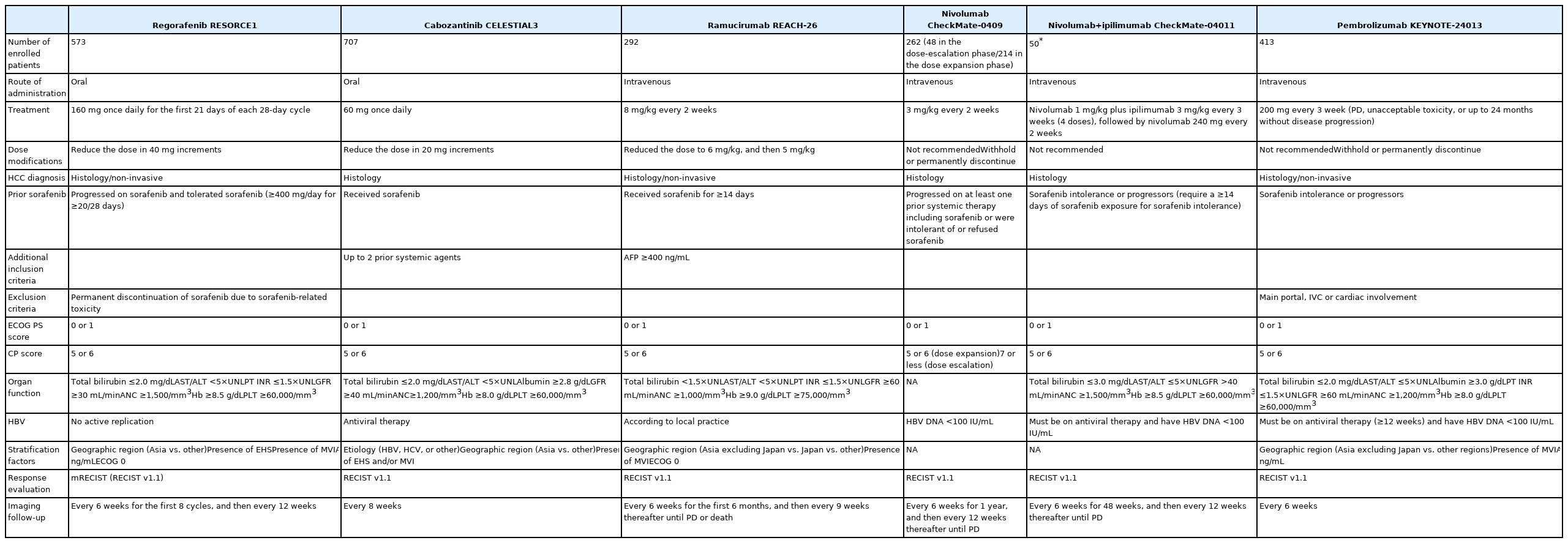
PDF - Several molecular-targeted agents have been tested as first- or second-line therapies for hepatocellular carcinoma (HCC) but failed to improve clinical outcomes; sorafenib has been the only approved systemic agent for treating HCC for almost 10 years. Regorafenib resulted in a significant improvement in overall survival and thus was approved for HCC patients previously treated with sorafenib. Subsequently, cabozantinib and ramucirumab demonstrated superior overall survival compared with placebos in phase III clinical trials. Immune checkpoint inhibitors such as nivolumab with or without ipilimumab and pembrolizumab are also available in some countries for patients who are unresponsive to sorafenib. Some second-line agents are available for patients who are unresponsive to sorafenib; however, little is known about the considerations for selecting appropriate secondline systemic agents. Hence, this study aimed to review the current and future perspectives of second-line systemic agents.
-
Citations
Citations to this article as recorded by- Expression of Peptidyl Arginine Deiminase 2 Is Closely Associated with Recurrence in Patients with Hepatocellular Carcinoma
Sunho Uhm, Yoon Cho, Ji-Young Choe, Ji Park, Min-Jeong Kim, Won-Ho Han, Junyong Lee, Jung Lee, Dong Shin, Jae Soh, Hyun Lim, Ho Kang, Sung-Hoon Moon, Sung-Eun Kim
Diagnostics.2023; 13(4): 659. CrossRef - Expert consensus on the management of adverse events in patients receiving lenvatinib for hepatocellular carcinoma
Bo Hyun Kim, Su Jong Yu, Wonseok Kang, Sung Bum Cho, Soo Young Park, Seung Up Kim, Do Young Kim
Journal of Gastroenterology and Hepatology.2022; 37(3): 428. CrossRef
- Expression of Peptidyl Arginine Deiminase 2 Is Closely Associated with Recurrence in Patients with Hepatocellular Carcinoma

Case Report
- Nivolumab for Advanced Hepatocellular Carcinoma with Multiple Lung Metastases after Sorafenib Failure
- Jaewoong Kim, Jin Won Chang, Jun Yong Park
- J Liver Cancer. 2020;20(1):72-77. Published online March 31, 2020
- DOI: https://doi.org/10.17998/jlc.20.1.72
- 4,851 Views
- 143 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
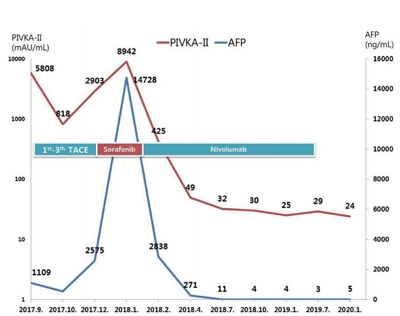
PDF - Over the past decade, standard first-line systemic treatment of advanced hepatocellular carcinoma (HCC) has been based on sorafenib, a multi-kinase inhibitor. Regorafenib, another tyrosine kinase inhibitor, is the only second-line therapy that has been globally approved after progression under sorafenib treatment. Recently, immunotherapeutic agents have emerged as promising treatment options in many different malignancies, including advanced HCC. Nivolumab is the first immunotherapy approved by the Food and Drug Administration for use in HCC patients with advanced-stage second-line after sorafenib failure. In this report, a case of advanced HCC with multiple lung metastases in which a complete response and maintained progression-free status was achieved with nivolumab, following the failure of transarterial chemoembolization and sorafenib is presented. We hope this report may help expand the clinical application of second-line treatment.
-
Citations
Citations to this article as recorded by- Infiltrative hepatocellular carcinoma with multiple lung metastasis completely cured using nivolumab: a case report
Ji Eun Han, Hyo Jung Cho, Soon Sun Kim, Jae Youn Cheong
Journal of Liver Cancer.2021; 21(2): 169. CrossRef
- Infiltrative hepatocellular carcinoma with multiple lung metastasis completely cured using nivolumab: a case report

Review Articles
- Deciphering and Reversing Immunosuppressive Cells in the Treatment of Hepatocellular Carcinoma
- Su Jong Yu, Tim F. Greten
- J Liver Cancer. 2020;20(1):1-16. Published online March 31, 2020
- DOI: https://doi.org/10.17998/jlc.20.1.1
- 7,111 Views
- 197 Downloads
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF - Use of immune checkpoint inhibitors (ICIs) in hepatocellular carcinoma (HCC) has been partially successful. However, most HCC patients do not respond to immunotherapy. HCC has been shown to induce several immune suppressor mechanisms in patients. These suppressor mechanisms include involvement of myeloid-derived suppressor cells, regulatory T-cells, functionally impaired dendritic cells (DCs), neutrophils, monocytes, and tumor associated macrophages. The accumulation of immunosuppressive cells may lead to an immunosuppressive tumor microenvironment as well as the dense fibrotic stroma which may contribute to immune tolerance. Our laboratory has been investigating different cellular mechanisms of immune suppression in HCC patients. In vitro as well as in vivo studies have demonstrated that abrogation of the suppressor cells enhances or unmasks tumor-specific antitumor immune responses. Two or three effective systemic therapies including ICIs and/or molecular targeted therapies and the addition of innovative combination therapies targeting immune suppressor cells may lead to increased immune recognition with a greater tumor response. We reviewed the literature for the latest research on immune suppressor cells in HCC, and here we provide a comprehensive summary of the recent studies in this field.
-
Citations
Citations to this article as recorded by- Higher Number of Tumor-Infiltrating PD-L1+ Cells Is Related to Better Response to Multikinase Inhibitors in Hepatocellular Carcinoma
Ji Won Han, Ji Hoon Kim, Dong Hyun Kim, Jeong Won Jang, Si Hyun Bae, Jong Young Choi, Seung Kew Yoon, Jaegyoon Ahn, Hyun Yang, Pil Soo Sung
Diagnostics.2023; 13(8): 1453. CrossRef - Immune checkpoint inhibitors in HCC: Cellular, molecular and systemic data
Uasim Harkus, Miriam Wankell, Pranavan Palamuthusingam, Craig McFarlane, Lionel Hebbard
Seminars in Cancer Biology.2022; 86: 799. CrossRef - Crosstalk between tumor-associated macrophages and neighboring cells in hepatocellular carcinoma
Pil Soo Sung
Clinical and Molecular Hepatology.2022; 28(3): 333. CrossRef
- Higher Number of Tumor-Infiltrating PD-L1+ Cells Is Related to Better Response to Multikinase Inhibitors in Hepatocellular Carcinoma

- Recent Advances and Future Directions in Immunotherapeutics for Hepatocellular Carcinoma
- Yuri Cho, Jimin Han, Won Kim
- J Liver Cancer. 2019;19(1):1-11. Published online March 31, 2019
- DOI: https://doi.org/10.17998/jlc.19.1.1

- 5,410 Views
- 149 Downloads
- 5 Citations
-
 Abstract
Abstract
 PDF
PDF - Systemic target therapeutic drugs, such as sorafenib, lenvatinib, or regorafenib are the only drugs that are known to be effective against advanced hepatocellular carcinoma (HCC). However, these agents show a limited efficacy in killing residual tumors. Immunotherapy is an alternative approach to this treatment and has been used to successfully treat different cancers, including HCC. HCC is an inflammation-induced cancer and represents a very interesting target for immunotherapeutics. Immunotherapies aim to reverse the immune tolerance and suppression found in tumor microenvironments and include approaches, such as adoptive cell therapy, immune checkpoint inhibition, and cancer vaccination. Adoptive cell therapy uses autologous natural killer or cytokine-induced killer cells by cultivating them ex vivo and subsequently reinfusing them into the patient. Immune checkpoint inhibitors reactivate tumorspecific T cells by suppressing checkpoint-mediated inhibitory signaling. Cancer vaccination induces a tumor-specific immune response by activating effector T lymphocytes. A wide range of potential immunotherapy-related adverse events occur; therefore, a multidisciplinary collaborative management is required across the clinical spectrum. This review summarizes the current status of immunotherapy for HCC and provides a perspective on its future applications.
-
Citations
Citations to this article as recorded by- Exploring the potential of Toxoplasma gondii in drug development and as a delivery system
Chanjin Yoon, Yu Seong Ham, Woo Jin Gil, Chul-Su Yang
Experimental & Molecular Medicine.2024; 56(2): 289. CrossRef - Comparison of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma with portal vein tumor thrombosis
Jeayeon Park, Yun Bin Lee, Yunmi Ko, Youngsu Park, Hyunjae Shin, Moon Haeng Hur, Min Kyung Park, Dae-Won Lee, Eun Ju Cho, Kyung-Hun Lee, Jeong-Hoon Lee, Su Jong Yu, Tae-Yong Kim, Yoon Jun Kim, Tae-You Kim, Jung-Hwan Yoon
Journal of Liver Cancer.2024; 24(1): 81. CrossRef - Revamping the innate or innate-like immune cell-based therapy for hepatocellular carcinoma: new mechanistic insights and advanced opportunities
Disha D. Shah, Bhavarth P. Dave, Parv A. Patel, Mehul R. Chorawala, Vishvas N. Patel, Palak A. Shah, Manish P. Patel
Medical Oncology.2023;[Epub] CrossRef - Differences between exhausted CD8+ T cells in hepatocellular carcinoma patients with and without uremia
Chen Xiaohong, Zou Jianzhou, Shen Bo, Lv Wenlv, Cao Xuesen, Xiang Fangfang
Canadian Journal of Physiology and Pharmacology.2021; 99(4): 395. CrossRef - Nivolumab for Advanced Hepatocellular Carcinoma with Multiple Lung Metastases after Sorafenib Failure
Jaewoong Kim, Jin Won Chang, Jun Yong Park
Journal of Liver Cancer.2020; 20(1): 72. CrossRef
- Exploring the potential of Toxoplasma gondii in drug development and as a delivery system

Case Report
- A Case of Management for Advanced Hepatocellular Carcinoma with Extrahepatic Metastasis by Autologous Natural Killer Cells Combined with Immune Checkpoint Inhibitor
- Woo, Ara , Kim, Eun Ju , Shin, Sun Young , Jeon, Hong Jae , Park, Hana , Chon, Young Eun , Lee, Yun Bin , Hwang, Seong Gyu , Rim, Kyu Sung , Lee, Joo Ho
- J Liver Cancer. 2018;18(1):67-74. Published online March 31, 2018
- DOI: https://doi.org/10.17998/jlc.18.1.67
- 2,017 Views
- 67 Downloads
-
 Abstract
Abstract
 PDF
PDF - Hepatocellular carcinoma (HCC) has extremely poor prognosis. Immunotherapy has emerged as a new treatment for a number of cancers. Adoptive immunotherapy is one of the important cancer immunotherapy, which relies on the various lymphocytes including cytotoxic T lymphocytes, natural killer (NK) and cytokine induced killer cells. Also, there has been advance in techniques of NK cell activation to more effectively kill the cancer cells. Of note, recently the blocking antibodies targeting programmed cell death protein 1 (PD-1) have shown promising results in diverse cancers including HCC. We report our recent experience of a patient accompanying advanced HCC with extrahepatic metastases. Disease progression had occurred after sorafenib administration, while the patient showed local tumor control and tumor marker decrease by NK cell immunotherapy combined with PD-1 inhibitor therapy. Though, there was no definite survival advantage due to impaired liver function, which might be caused by treatment related toxicities as well as cancer progression.

Review Article
- Systemic Therapy for Advanced Hepatocellular Carcinoma: Targeted Therapy and Immunotherapy
- Kim, Bo Hyun , Park, Joong Won
- J Liver Cancer. 2018;18(1):17-22. Published online March 31, 2018
- DOI: https://doi.org/10.17998/jlc.18.1.17
- 2,320 Views
- 80 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
PDF - Systemic therapy for hepatocellular carcinoma (HCC) has markedly changed since 2007, with the approval of sorafenib. Sorafenib improved the overall survival of patients with advanced HCC; however, the modest efficacy and toxicity of this therapy present unmet needs. Subsequently, a variety of molecular targeted agents have been tested as first-line or secondline therapies but have failed, and sorafenib has remained the only approved systemic agent for almost 10 years. Recently, regorafenib significantly improved overall survival and was approved for patients with HCC who have been previously treated with sorafenib. Nivolumab, a programmed death protein-1 inhibitor, was also approved as second-line therapy, based on remarkable response rates.
-
Citations
Citations to this article as recorded by- Analysis of Existing Guidelines and Randomized, Controlled, Clinical Trials for Development of [Guideline of Clinical Trial on Herbal Medicinal Product for Liver Cancer]
Ga-jin Han, Dong-hun Kim, Eun-joo Park, Sin Seong, Sung-su Kim, Jung-tae Leem
The Journal of Internal Korean Medicine.2019; 40(1): 89. CrossRef
- Analysis of Existing Guidelines and Randomized, Controlled, Clinical Trials for Development of [Guideline of Clinical Trial on Herbal Medicinal Product for Liver Cancer]


 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION

 First
First Prev
Prev



 Follow JLC on Twitter
Follow JLC on Twitter