Articles
- Page Path
- HOME > J Liver Cancer > Volume 24(1); 2024 > Article
-
Original Article
Comparison of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma with portal vein tumor thrombosis -
Jeayeon Park1,2
 , Yun Bin Lee1,2
, Yun Bin Lee1,2 , Yunmi Ko1,2
, Yunmi Ko1,2 , Youngsu Park1,2
, Youngsu Park1,2 , Hyunjae Shin1,2
, Hyunjae Shin1,2 , Moon Haeng Hur1,2
, Moon Haeng Hur1,2 , Min Kyung Park1,2
, Min Kyung Park1,2 , Dae-Won Lee3
, Dae-Won Lee3 , Eun Ju Cho1,2
, Eun Ju Cho1,2 , Kyung-Hun Lee3
, Kyung-Hun Lee3 , Jeong-Hoon Lee1,2
, Jeong-Hoon Lee1,2 , Su Jong Yu1,2
, Su Jong Yu1,2 , Tae-Yong Kim3
, Tae-Yong Kim3 , Yoon Jun Kim1,2
, Yoon Jun Kim1,2 , Tae-You Kim3
, Tae-You Kim3 , Jung-Hwan Yoon1,2
, Jung-Hwan Yoon1,2
-
Journal of Liver Cancer 2024;24(1):81-91.
DOI: https://doi.org/10.17998/jlc.2023.12.25
Published online: January 19, 2024
1Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
2Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
3Cancer Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- Corresponding author: Yun Bin Lee, Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea E-mail: yblee@snu.ac.kr
© 2024 The Korean Liver Cancer Association.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 992 Views
- 133 Downloads
Abstract
-
Background/Aim
- Atezolizumab plus bevacizumab and lenvatinib are currently available as first-line therapy for the treatment of unresectable hepatocellular carcinoma (HCC). However, comparative efficacy studies are still limited. This study aimed to investigate the effectiveness of these treatments in HCC patients with portal vein tumor thrombosis (PVTT).
-
Methods
- We retrospectively included patients who received either atezolizumab plus bevacizumab or lenvatinib as first-line systemic therapy for HCC with PVTT. Primary endpoint was overall survival (OS), and secondary endpoints included progressionfree survival (PFS) and disease control rate (DCR) determined by response evaluation criteria in solid tumors, version 1.1.
-
Results
- A total of 52 patients were included: 30 received atezolizumab plus bevacizumab and 22 received lenvatinib. The median follow-up duration was 6.4 months (interquartile range, 3.9-9.8). The median OS was 10.8 months (95% confidence interval [CI], 5.7 to not estimated) with atezolizumab plus bevacizumab and 5.8 months (95% CI, 4.8 to not estimated) with lenvatinib (P=0.26 by log-rank test). There was no statistically significant difference in OS (adjusted hazard ratio [aHR], 0.71; 95% CI, 0.34-1.49; P=0.37). The median PFS was similar (P=0.63 by log-rank test), with 4.1 months (95% CI, 3.3-7.7) for atezolizumab plus bevacizumab and 4.3 months (95% CI, 2.6-5.8) for lenvatinib (aHR, 0.93; 95% CI, 0.51-1.69; P=0.80). HRs were similar after inverse probability treatment weighting. The DCRs were 23.3% and 18.2% in patients receiving atezolizumab plus bevacizumab and lenvatinib, respectively (P=0.74).
-
Conclusion
- The effectiveness of atezolizumab plus bevacizumab and lenvatinib was comparable for the treatment of HCC with PVTT.
- Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide.1 With the approval of immune checkpoint inhibitor-based treatments as standard systemic therapy, the range of treatment options for advanced HCC has expanded, leading to improved outcomes for HCC patients.2-4 The phase III IMbrave 150 trial showed significantly improved overall survival (OS) and progression-free survival (PFS) in patients receiving the combination therapy with atezolizumab plus bevacizumab compared with patients receiving sorafenib. Thus, the combination therapy with atezolizumab plus bevacizumab has been established as the first-line treatment for unresectable HCC.4
- In the REFLECT trial, lenvatinib was showed to be non-inferior to sorafenib in terms of OS and was approved as a first-line treatment for unresectable HCC.5 Additionally, lenvatinib is the only agent among various tyrosine kinase inhibitors that has demonstrated equivalent efficacy compared to sorafenib. Several previous studies have compared atezolizumab plus bevacizumab with lenvatinib, but the results have been inconsistent.6,7 In a real-world, multicenter study conducted in South Korea, there was no significant difference in OS and PFS between the two treatments.6 By contrast, in another study including patients with non-alcoholic fatty liver disease-related HCC, lenvatinib treatment was associated with a significant survival benefit compared to the combination treatment with atezolizumab plus bevacizumab.7 Therefore, additional studies are needed to compare the efficacy of the combination of atezolizumab plus bevacizumab and lenvatinib in various clinical conditions.
- HCC with portal vein tumor thrombosis (PVTT) is known to be associated with an aggressive disease course, reduced liver function reserve, and higher rates of recurrence after treatment, resulting in worse OS.8-10 Therefore, studies are warranted to determine the appropriate treatment strategies for HCC patients with PVTT. In this study, we aimed to compare the effectiveness and safety of atezolizumab plus bevacizumab and lenvatinib as first-line therapy for the treatment of HCC with PVTT.
INTRODUCTION
- Study population
- We retrospectively included consecutive patients who received either atezolizumab plus bevacizumab or lenvatinib as first-line systemic therapy for the treatment of HCC with PVTT between October 2020 and July 2022 at Seoul National University Hospital, Seoul, Korea. HCC was diagnosed radiologically and/or histologically according to the latest updated guidelines.11-13 This study conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. H-1805-055-944). The requirement to obtain informed consent from patients was waived owing to the retrospective nature of the study.
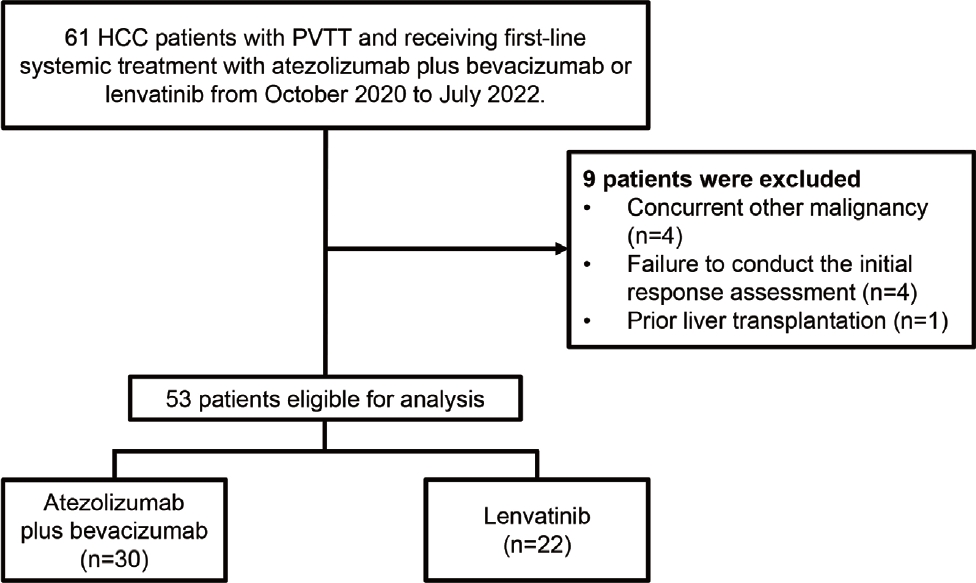
- Patients were included in the study if they met the following eligibility criteria: 1) age of >18 years, 2) unequivocal diagnosis of HCC based on radiological and/or histological findings, 3) presence of PVTT, 4) treatment with atezolizumab plus bevacizumab or lenvatinib as first-line systemic therapy, and 5) Eastern Cooperative Oncology Group performance status of 0 or 1. Patients who met any of the following criteria were excluded: 1) presence of other concurrent malignancy, 2) failure to conduct the initial response assessment after administration of atezolizumab plus bevacizumab or lenvatinib, or 3) prior recipient of liver transplantation.
- Treatment regimens
- Study patients were prescribed either atezolizumab plus bevacizumab or lenvatinib based on the patient's preference and the attending physician's advice considering the clinical situation. The atezolizumab plus bevacizumab regimen was administered intravenously, with atezolizumab given at a dose of 1,200 mg, followed by bevacizumab at a dose of 15 mg/kg, both on the same day every 3 weeks. Lenvatinib was administered at a dose of 12 mg orally once daily for patients who weighed 60 kg or more, and at a dose of 8 mg orally once daily for patients who weighed less than 60 kg. Treatment with atezolizumab plus bevacizumab or lenvatinib was discontinued in patients who developed disease progression, experienced intolerable toxicity, or developed liver failure.
- Assessments and outcomes
- The classification of PVTT was based on the Liver Cancer Study Group of Japan classification as follows: Vp1 represents PVTT located distal to, but not within the second-order branches of the left and right portal veins, Vp2 indicates PVTT located within the second-order branches of the portal veins, Vp3 corresponds to PVTT located within the first-order branches of the portal veins, and Vp4 denotes PVTT located in the main trunk of the portal vein or PVTT in the contralateral portal vein branch, or both.14 Esophageal varices were graded according to the criteria proposed by the Japanese Research Society for Portal Hypertension on endoscopic examination.15 High-risk varices were defined as esophageal varices graded 2 or higher, those with a red color sign, or the presence of gastric varices.16
- The index date was defined as the date on which the patient started treatment with atezolizumab plus bevacizumab or lenvatinib. Patients were censored at the date of the last follow-up, death, or data cutoff (April 30, 2023), whichever came first. The primary endpoint was OS. The secondary endpoints were PFS, time-to-progression (TTP), disease control rate (DCR), and safety. Radiological response to the treatment was assessed every 6 to 12 weeks based on dynamic computed tomography or magnetic resonance imaging, in accordance with the response evaluation criteria in solid tumors version 1.1.17 Tumor markers, including serum levels of alpha-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II) were measured every 6 to 12 weeks.11,12,18 Safety was evaluated based on the National Cancer Institute common terminology criteria for adverse events, version 5.0.
- Statistical analysis
- Baseline clinical and demographic characteristics were compared between the two groups. Categorical variables were presented as frequencies (%) and continuous variables as medians with interquartile ranges (IQRs). To compare categorical variables, Pearson’s chi-square test or Fisher’s exact test was utilized, while continuous variables were compared using Student’s t-test or Mann-Whitney U test. Inverse probability of treatment weighting (IPTW) was applied to minimize potential confounding19 in the propensity score calculation by incorporating variables such as age, sex, presence or absence of liver cirrhosis, esophageal or gastric varices, fibrosis-4 index, Child-Pugh score, serum levels of AFP and PIVKA-II, extent of PVTT, presence or absence of hepatic vein invasion of HCC, and presence or absence of lymph node or extrahepatic metastasis. The Kaplan-Meier estimator and log-rank test were used to analyze OS, PFS, and TTP. For risk factor analysis, multivariable analysis was performed using Cox regression analysis for covariables that were found to be significant in the univariable analysis or had a low degree of collinearity.
- All statistical analyses were performed using R version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria). P-values less than 0.05 were considered to indicate statistically significant differences.
PATIENTS AND METHODS
- Study population
- A total of 52 patients were included in this study: 30 received atezolizumab plus bevacizumab and 22 received lenvatinib (Fig. 1). The median follow-up duration was 6.4 months (IQR, 3.9-9.8). The baseline characteristics of the study population are presented in Table 1. There was no significant difference in age and sex between the two groups (P=0.93 and P=1.00, respectively). Hepatitis B was the most common etiology of HCC in both groups, and there was no statistically significant difference between the groups (P=1.00). The atezolizumab plus bevacizumab group tended to show a lower proportion of esophageal varices than the lenvatinib group at baseline (53.3% and 59.1%, respectively), while there was no statistically significant difference (P=0.90). The proportion of patients with Child-Pugh score of 5 and Vp1 PVTT tended to be higher in the atezolizumab plus bevacizumab group than the lenvatinib group (P=0.58 and P=0.09, respectively).
- OS
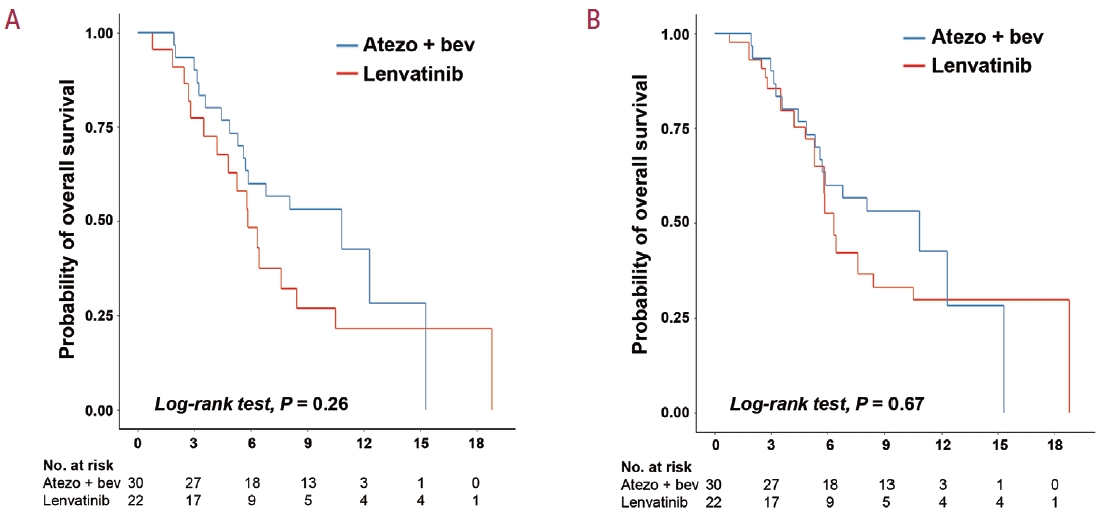
- During the follow-up period, 34 of 52 patients died. Seventeen (56.7%) patients are the atezolizumab plus bevacizumab group and 17 (77.3%) patients are in the lenvatinib group. The median OS was 10.8 months (95% confidence interval [CI], 5.7 to not estimated) in the atezolizumab plus bevacizumab group and 5.8 months (95% CI, 4.8 to not estimated) in the lenvatinib group (P=0.26, by log-rank test) (Fig. 2). There was no statistically significant difference in OS between the two groups (atezolizumab plus bevacizumab group vs. lenvatinib group; hazard ratio [HR], 0.67; 95% CI, 0.34-1.34; P=0.26) (Fig. 2A). Even after IPTW, OS was not significantly different between the two groups (HR, 0.84; 95% CI, 0.38-1.86; P=0.67) (Fig. 2B). Multivariable analyses showed that there were no significant differences in OS between the two groups, both before IPTW (adjusted HR [aHR], 0.71; 95% CI, 0.34-1.49; P=0.37; Table 2) and after IPTW (aHR, 0.74; 95% CI, 0.37-1.49; P=0.40; Table 2). In the multivariable analyses both before and after IPTW, higher Child-Pugh scores were significantly associated with decreased OS (Table 2). Among these 40 patients with Vp3-Vp4 PVTT, 32 patients died during the follow-up period: 15 (50.0%) in the atezolizumab plus bevacizumab group and 17 (77.3%) in the lenvatinib group. The median OS was 6.3 months (95% CI, 5.3 to not estimated) in the atezolizumab plus bevacizumab group and 5.8 months (95% CI, 4.2-10.5) in the lenvatinib group (P=0.50, by log-rank test; Supplementary Fig. 1A). There were no statistically significant differences in OS between the atezolizumab plus bevacizumab group and the lenvatinib group (HR, 0.80; 95% CI, 0.39-1.63; P=0.50; Supplementary Fig. 1A), and this finding remained consistent even after IPTW (HR 0.87; 95% CI, 0.44-1.73; P=0.70; Supplementary Fig. 1B).
- PFS and TTP
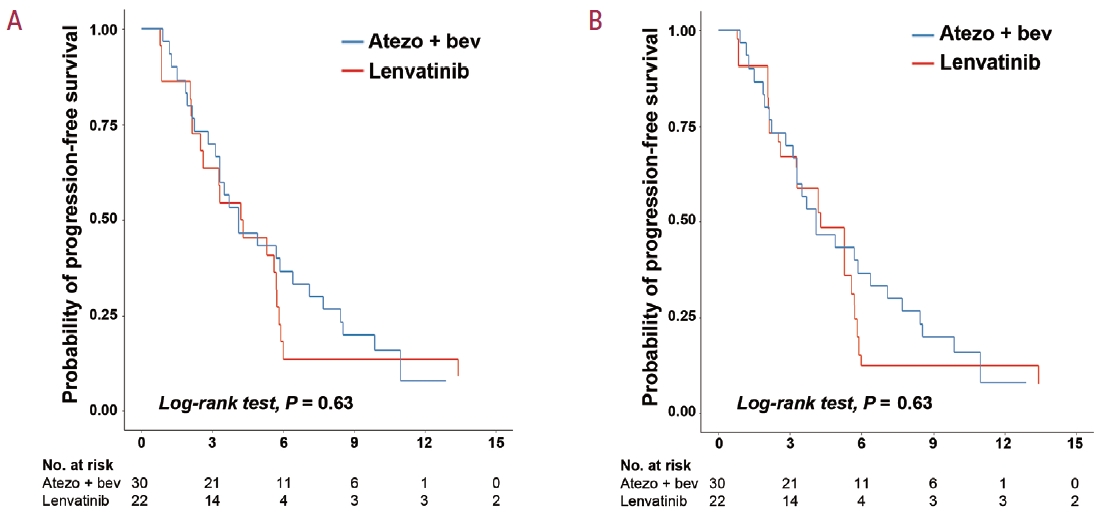
- Forty-five (86.5%) patients showed disease progression or died: 25 (83.3%) patients in the atezolizumab plus bevacizumab group and 20 (90.9%) patients in the lenvatinib group. The median PFS was similar between the two treatment groups, with a median of 4.1 months (95% CI, 3.3-7.7) for atezolizumab plus bevacizumab and 4.3 months (95% CI, 2.6-5.8) for lenvatinib (P=0.63, by log-rank test; Fig. 3A). There was no statistically significant difference in PFS between the two groups, both before IPTW (HR, 0.86; 95% CI, 0.47-1.57, P=0.63; Fig. 3A) and after IPTW (HR, 0.86; 95% CI, 0.48-1.57; P=0.63; Fig. 3B). In multivariable analyses, no significant differences were observed in PFS between the two groups before IPTW (aHR, 0.93; 95% CI, 0.51-1.69; P=0.80) and after IPTW (aHR, 0.87; 95% CI, 0.49-1.56; P=0.64; Supplementary Table 1). Thirty-six (90.0%) patients with Vp3-Vp4 PVTT showed disease progression or died: 18 (90.0%) in the atezolizumab plus bevacizumab group and 18 (90.0%) in the lenvatinib group. The median PFS was also similar between the two treatment groups, with a median of 3.6 months (95% CI, 2.8-6.4) for the atezolizumab plus bevacizumab group and 3.8 months (95% CI, 2.5-5.9) for the lenvatinib group (P=0.90, by log-rank test; Supplementary Fig. 2A). There were no statistically significant differences in PFS between the two groups before IPTW (HR 1.04; 95% CI, 0.54-2.03; P=0.90; Supplementary Fig. 2A) and after IPTW (HR 1.06; 95% CI, 0.55-2.03; P=0.90; Supplementary Fig. 2B).
- Disease progression was observed in 38 of 52 patients: 21 (70.0%) patients in the atezolizumab plus bevacizumab group and 17 (77.3%) patients in the lenvatinib group. The median TTP was also similar between the two treatment groups, with a median of 4.1 months (95% CI, 2.6-6.0) for atezolizumab plus bevacizumab and 4.1 months (95% CI, 3.3-9.9) for lenvatinib (P=0.56, by log-rank test; Supplementary Fig. 3). TTP between the two groups showed no statistically significant difference before IPTW (atezolizumab plus bevacizumab group vs. lenvatinib group; HR, 0.82; 95% CI, 0.43-1.58; P=0.56; Supplementary Fig. 3A). There was also no significant difference between the two groups after IPTW (HR, 0.79; 95% CI, 0.42-1.52; P=0.49; Supplementary Fig. 3B). Multivariable analyses showed that there was no significant difference in TTP between the two groups before IPTW (aHR, 0.90; 95% CI, 0.45-1.78; P=0.75) and after IPTW (aHR, 0.83; 95% CI, 0.44-1.58; P=0.57; Supplementary Table 2). Among 40 patients with Vp3-Vp4 PVTT, 29 (72.5%) experienced disease progression: 14 (70.0%) in the atezolizumab plus bevacizumab group and 15 (75.0%) in the lenvatinib group. The median TTP was similar between the two treatment groups, with a median of 3.7 months (95% CI, 3.1 to not estimated) for the atezolizumab plus bevacizumab group and 4.3 months (95% CI, 2.6-13.4) for the lenvatinib group (P=0.90, by log-rank test; Supplementary Fig. 4A). There were no statistically significant differences in TTP between the groups before IPTW (HR 0.95; 95% CI, 0.45-2.00; P=0.90; Supplementary Fig. 4A) and after IPTW (HR 0.94; 95% CI, 0.45-1.98; P=0.90; Supplementary Fig. 4B).
- Treatment responses
- Among the 52 patients assessed for a tumor response, seven (23.3%) patients in the atezolizumab plus bevacizumab group and four (18.2%) patients in the lenvatinib group showed stable disease (Table 3). Complete and partial responses were not achieved in any patient. The DCR tended to be higher in the atezolizumab plus bevacizumab group (23.3%) than that in the lenvatinib group (18.2%). However, the difference between the two groups was not statistically significant (P=0.74; Table 3). In the subgroup analysis among patients with Vp3-Vp4 PVTT, there was no significant difference in DCR between the two groups (P=1.00; Supplementary Table 3).
- Among 41 patients who showed progressive disease after treatment with the combination of atezolizumab plus bevacizumab (23 patients) or lenvatinib (18 patients), 22 patients (15 [65.2%] in the atezolizumab plus bevacizumab group and seven [38.9%] in the lenvatinib group) received sorafenib as the subsequent chemotherapy and 19 patients (eight [34.8%] in the atezolizumab plus bevacizumab group and 11 [61.1%] in the lenvatinib group) received only best supportive care without any additional chemotherapy.
- Safety
- All adverse events in study patients are summarized in Supplementary Table 4. Adverse events of any grade related to treatment occurred in 22 of 52 patients: eight (26.7%) patients in the atezolizumab plus bevacizumab group and 14 (63.6%) in the lenvatinib group (P=0.02). Five (9.6%) patients experienced grade 3 or higher adverse events: three (10.0%) patients in the atezolizumab plus bevacizumab group and two (9.1%) in the lenvatinib group (P=1.00). The most common adverse events were skin rash (four [13.3%] patients) in the atezolizumab plus bevacizumab group and skin rash (four [18.2%] patients), hypertension (four [18.2%] patients), and diarrhea (four [18.2%] patients) in the lenvatinib group. All adverse events improved and resolved with supportive care. In particular, the incidences of hypertension and diarrhea were significantly higher in the lenvatinib group than in the atezolizumab plus bevacizumab group (P=0.03 for hypertension; P=0.03 for diarrhea; Supplementary Table 4). The Grade 3 or higher severe adverse events in both groups was bleeding. Two (6.7%) patients in the atezolizumab plus bevacizumab group experienced gastrointestinal (GI) tract bleeding: one with ulcer bleeding and one with variceal bleeding. One (4.5%) patient in the lenvatinib group developed ulcer bleeding.
- We assessed the reasons for discontinuing treatment of the combination of atezolizumab plus bevacizumab or lenvatinib treatments in the study patients. In the atezolizumab plus bevacizumab group, three (10.0%) patients discontinued due to adverse events. Among them, two patients who discontinued treatment because of GI bleeding resumed treatment without dose modification thereafter. However, one patient who discontinued due to febrile neutropenia terminated treatment, because the patient showed disease progression. In the lenvatinib group, two (9.5%) patients discontinued treatment due to GI bleeding, and treatment was terminated after disease progression was confirmed.
RESULTS
- The effectiveness of atezolizumab plus bevacizumab and lenvatinib was comparable for the treatment of HCC with PVTT in our current study. OS, PFS, and TTP were not significantly different between patients receiving atezolizumab plus bevacizumab and lenvatinib. These findings were similar even after balancing patient characteristics using the IPTW. While OS was longer in patients receiving atezolizumab plus bevacizumab (10.8 months) than in patients receiving lenvatinib (5.8 months), the difference was not statistically significant. Although the incidences of adverse events of any grade were similar between the two treatment regimens, it is noteworthy that the proportions of patients experiencing hypertension and diarrhea were significantly higher among patients treated with lenvatinib.
- Based on the results of the REFLECT trial, sorafenib and lenvatinib showed comparable efficacy, leading to the anticipation that the combination of atezolizumab plus bevacizumab would also result in longer OS and PFS compared to lenvatinib.4,5 However, heterogeneous findings were found across various studies.6,7 Although the sample size of our study was small, we compared the two first-line systemic treatments in HCC patients with PVTT and found comparable effectiveness between atezolizumab plus bevacizumab and lenvatinib. After adjustment for baseline characteristics using IPTW, the gap in survival curves of atezolizumab plus bevacizumab and lenvatinib has narrowed further. There are some potential mechanisms that can explain these findings. Lenvatinib, through the inhibition of VEGF receptor and fibroblast growth factor receptor pathways, exhibits a more pleiotropic effect than sorafenib.20-24 Both tyrosine kinase inhibitors showed comparable anti-tumor activity in the immunodeficient Hepa1-6 tumor model. However, in the immunocompetent tumor model, lenvatinib was much more effective than sorafenib. Lenvatinib almost completely suppressed the in vivo growth of Hepa1‐6 tumors in immunocompetent mice, whereas sorafenib only slowed tumor growth compared to the non‐ treatment control. Thus, lenvatinib exhibited significantly more potent antitumor activity than sorafenib in the immunocompetent Hepa1‐6 tumor model.22 Moreover, preclinical studies have confirmed its impact on the tumor microenvironment, promoting CD8+ T cell infiltration while reducing the number of tumor-associated macrophages.21,24 Atezolizumab plus bevacizumab also facilitates CD8+ T cell infiltration into regions harboring cancer cells, activating cytotoxic effects and inducing tumor cell apoptosis.25 Therefore, increased CD8+ T cell infiltration in these cancerous regions could be a common anti-cancer mechanism shared by atezolizumab plus bevacizumab and lenvatinib, providing a plausible explanation for the comparable efficacy observed between the two treatments.
- In both the atezolizumab plus bevacizumab and lenvatinib groups, none of the patients showed a complete or partial response. The lack of favorable responses in this study, when compared to previous studies, including the IMbrave150 trial and the REFLECT trials, can likely be attributed to the inclusion of only HCC patients with PVTT, a factor associated with an unfavorable prognosis.8-10,13,26 Additionally, the higher proportion of patients with Child-Pugh score of six in our current study compared to the previous studies further contributes to the results.27 Therefore, only stable disease or progressive disease was observed as the best treatment response. This is especially highlighted by the results that Vp4 PVTT was identified as an independent risk factor for poor OS.
- Regarding adverse events, patients in the two groups developed different types of bleeding during the study period. One patient in the atezolizumab plus bevacizumab group developed variceal bleeding, while three patients in the lenvatinib group experienced gastrointestinal bleeding other than variceal bleeding. The occurrence of different types of bleeding may be attributed to the distinct mechanisms of the two regimens. Lenvatinib targets VEGF receptors, whereas bevacizumab inhibits circulating VEGF.28 Although both mechanisms impair hemostatic function, the specific inhibition of circulating VEGF leads to reduced capillary bed density in the liver and increased pressure in the reticuloendothelial system, resulting in exacerbated varicose veins.28,29 Consequently, it is postulated that variceal bleeding is more prone to occur in patients receiving atezolizumab plus bevacizumab than in those receiving lenvatinib.
- The retrospective nature and small sample size are some of the limitations of this study. The combination therapy with atezolizumab plus bevacizumab was approved and clinically applied in South Korea in 2020. Moreover, the current study included only HCC patients with PVTT, leading to a short follow-up period and a limited number of subjects. To overcome potential biases that may arise from these conditions, we applied IPTW to adjust for between-group baseline differences, and similar results were observed even after IPTW. Hence, further long-term follow-up studies in a larger sample population are essential to validate our results.
- In conclusion, atezolizumab plus bevacizumab and lenvatinib showed comparable effectiveness as first-line systemic therapy in HCC patients with PVTT. Further studies with larger sample sizes and long-term follow-up periods are needed to validate these results.
DISCUSSION
-
Conflict of Interest
The authors have no conflicts to disclose.
-
Ethics Statement
This study conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-1805-055-944). The requirement to obtain informed consent from patients was waived owing to the retrospective nature of the study.
-
Funding Statement
This study was supported by the Scientific Research Fund of the Korean Liver Cancer Association (2021).
-
Data Availability
The data presented in this study are available upon request from the corresponding author.
-
Author Contribution
Conceptualization: JP, YBL, DWL
Data curation: JP, YBL, YK, YP, HS, MHH, MKP, EJC, KHL, JHL, SJY, TYK, YJK, TYK, JHY
Formal analysis: JP, YBL
Investigation: JP, YBL, YK, YP, HS, MHH, MKP, DWL, EJC, KHL, JHL, SJY, TYK, YJK, TYK, JHY
Methodology: JP, YBL
Project administration: YBL
Resources: JP
Supervision: JHY
Visualization: JP
Writing - original draft: JP, YBL
Writing - review & editing: JP, YBL, YK, YP, HS, MHH, MKP, DWL, EJC, KHL, JHL, SJY, TYK, YJK, TYK, JHY
Article information
Supplementary Material
Values are presented as median (range) or number (%).
IPTW, inverse probability of treatment weighting; SMD, standardized mean difference; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist-II; PVTT, portal vein tumor thrombosis; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; TARE, transarterial radioembolization.
| Variable |
Before IPTW |
After IPTW |
||||||
|---|---|---|---|---|---|---|---|---|
| Crude HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value | Crude HR(95% CI) | P-value | Adjusted HR(95% CI) | P-value | |
| Treatment | 0.26 | 0.37 | 0.67 | 0.40 | ||||
| Lenvatinib | 1* | 1* | 1* | 1* | ||||
| Atezolizumab plus bevacizumab | 0.67 (0.34-1.34) | 0.71 (0.34-1.49) | 0.84 (0.38-1.86) | 0.74 (0.37-1.49) | ||||
| Age (years) | 0.84 | 0.48 | ||||||
| <65 | 1* | 1* | ||||||
| ≥65 | 1.08 (0.53-2.20) | 1.31 (0.63-2.72) | ||||||
| Sex | 0.90 | 0.97 | ||||||
| Female | 1* | 1* | ||||||
| Male | 1.06 (0.44-2.57) | 1.02 (0.40-2.60) | ||||||
| Etiology of HCC | 0.63 | 0.66 | ||||||
| Other etiology | 1* | 1* | ||||||
| HBV or HCV | 0.75 (0.23-2.47) | 0.73 (0.17-3.05) | ||||||
| Esophageal varix | 0.14 | 0.11 | ||||||
| Absent | 1* | 1* | ||||||
| Present | 1.71 (0.83-3.52) | 1.88 (0.86-4.07) | ||||||
| Child-Pugh score | <0.001 | 0.003 | <0.001 | 0.003 | ||||
| A5 | 1* | 1* | 1* | 1* | ||||
| A6 | 4.42 (1.89-10.35) | 3.57 (0.74-1.90) | 5.17 (2.08-12.84) | 3.57 (0.74-1.90) | ||||
| AFP (ng/mL) | 0.68 | 0.44 | ||||||
| <200 | 1* | 1* | ||||||
| ≥200 | 1.16 (0.57-2.36) | 1.38 (0.61-3.13) | ||||||
| PIVKA-II (mAU/mL) | 0.28 | 0.17 | ||||||
| <1,000 | 1* | 1* | ||||||
| ≥1,000 | 1.47 (0.73-2.97) | 1.70 (0.80-3.62) | ||||||
| Number of hepatic masses | 0.82 | 0.55 | ||||||
| Single | 1* | 1* | ||||||
| Multiple | 0.92 (0.46-1.86) | 0.79 (0.37-1.70) | ||||||
| LN metastasis | 0.49 | |||||||
| Absent | 1* | 1* | 0.66 | |||||
| Present | 0.72 (0.72-1.87) | 0.77 (0.24-2.48) | ||||||
| Extrahepatic metastasis | 0.27 | |||||||
| Absent | 1* | 1* | 0.22 | |||||
| Present | 1.48 (0.74-2.97) | 1.61 (0.76-3.43) | ||||||
| Extent of PVTT | 0.01 | 0.08 | 0.01 | |||||
| No main PV invasion | 1* | 1* | 1* | 1* | 0.09 | |||
| Main PV invasion | 6.31 (1.51-26.44) | 3.69 (0.85-16.04) | 7.88 (1.53-40.70) | 4.45 (0.81-24.30) | ||||
| Hepatic vein invasion | 0.89 | 0.81 | ||||||
| Absent | 1* | 1* | ||||||
| Present | 0.90 (0.21-3.79) | 0.83 (0.17-4.00) | ||||||
OS, overall survival; IPTW, inverse probability of treatment weighting; HR, hazard ratio; CI, confidence interval; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist-II; LN, lymph node; PVTT, portal vein tumor thrombosis; PV, portal vein.
* Reference.
- 1. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology 2021;73 Suppl 1:4−13.ArticlePubMedPMCPDF
- 2. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid 2022;1:EVIDoa2100070. PubMed
- 3. Cho Y, Han J, Kim W. Recent advances and future directions in immunotherapeutics for hepatocellular carcinoma. J Liver Cancer 2019;19:1−11.ArticlePDF
- 4. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894−1905.ArticlePubMed
- 5. Yamashita T, Kudo M, Ikeda K, Izumi N, Tateishi R, Ikeda M, et al. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol 2020;55:113−122.ArticlePubMedPMCPDF
- 6. Kim BK, Cheon J, Kim H, Kang B, Ha Y, Kim DY, et al. Atezolizumab/ bevacizumab vs. lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: a real-world, multi-center study. Cancers (Basel) 2022;14:1747. ArticlePubMedPMC
- 7. Rimini M, Rimassa L, Ueshima K, Burgio V, Shigeo S, Tada T, et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: an international propensity score matching analysis. ESMO Open 2022;7:100591. PubMedPMC
- 8. Jin S, Choi WM, Shim JH, Lee D, Kim KM, Lim YS, et al. Subclassification of advanced-stage hepatocellular carcinoma with macrovascular invasion: combined transarterial chemoembolization and radiotherapy as an alternative first-line treatment. J Liver Cancer 2023;23:177−188.ArticlePubMedPMCPDF
- 9. Khan AR, Wei X, Xu X. Portal vein tumor thrombosis and hepatocellular carcinoma - the changing tides. J Hepatocell Carcinoma 2021;8:1089−1115.ArticlePubMedPMCPDF
- 10. Liu PH, Huo TI, Miksad RA. Hepatocellular carcinoma with portal vein tumor involvement: best management strategies. Semin Liver Dis 2018;38:242−251.ArticlePubMed
- 11. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358−380.ArticlePubMedPDF
- 12. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182−236.ArticlePubMed
- 13. Korean Liver Cancer Association (KLCA), National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. J Liver Cancer 2023;23:1−120.ArticlePubMedPMCPDF
- 14. Nevarez NM, Yopp AC. Challenging the treatment paradigm: selecting patients for surgical management of hepatocellular carcinoma with portal vein tumor thrombus. J Hepatocell Carcinoma 2021;8:851−860.ArticlePubMedPMCPDF
- 15. Idezuki Y. General rules for recording endoscopic findings of esophagogastric varices (1991). Japanese Society for Portal Hypertension. World J Surg 1995;19:420−422. discussion 423.ArticlePubMedPDF
- 16. Cunningham ME, Parastandeh-Chehr G, Cerocchi O, Wong DK, Patel K. Noninvasive predictors of high-risk varices in patients with non-cirrhotic portal hypertension. Can J Gastroenterol Hepatol 2019;2019:1808797. ArticlePubMedPMCPDF
- 17. Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer 2016;62:132−137.ArticlePubMedPMC
- 18. Korean Liver Cancer Association (KLCA), National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol 2022;23:1126−1240.ArticlePubMedPMCPDF
- 19. Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med 2016;35:5642−5655.ArticlePubMedPMCPDF
- 20. Dipasquale A, Marinello A, Santoro A. A comparison of lenvatinib versus sorafenib in the first-line treatment of unresectable hepatocellular carcinoma: selection criteria to guide physician's choice in a new therapeutic scenario. J Hepatocell Carcinoma 2021;8:241−251.ArticlePubMedPMCPDF
- 21. Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One 2019;14:e0212513.ArticlePubMedPMC
- 22. Kimura T, Kato Y, Ozawa Y, Kodama K, Ito J, Ichikawa K, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci 2018;109:3993−4002.ArticlePubMedPMCPDF
- 23. Kudo M. Lenvatinib may drastically change the treatment landscape of hepatocellular carcinoma. Liver Cancer 2018;7:1−19.ArticlePubMedPMCPDF
- 24. Takase N, Koma Y, Urakawa N, Nishio M, Arai N, Akiyama H, et al. NCAM- and FGF-2-mediated FGFR1 signaling in the tumor microenvironment of esophageal cancer regulates the survival and migration of tumor-associated macrophages and cancer cells. Cancer Lett 2016;380:47−58.ArticlePubMed
- 25. Kuwano A, Yada M, Miyazaki Y, Tanaka K, Kurosaka K, Ohishi Y, et al. Tumor‑infiltrating CD8+ T cells as a biomarker for chemotherapy efficacy in unresectable hepatocellular carcinoma. Oncol Lett 2023;25:259. ArticlePubMedPMC
- 26. Mukozu T, Nagai H, Matsui D, Mohri K, Watanabe G, Yoshimine N, et al. Adaptation of lenvatinib treatment in patients with hepatocellular carcinoma and portal vein tumor thrombosis. Cancer Chemother Pharmacol 2022;89:11−20.ArticlePubMedPDF
- 27. Lee SK, Kwon JH, Lee SW, Lee HL, Kim HY, Kim CW, et al. A realworld comparative analysis of atezolizumab plus bevacizumab and transarterial chemoembolization plus radiotherapy in hepatocellular carcinoma patients with portal vein tumor thrombosis. Cancers (Basel) 2023;15:4423. ArticlePubMedPMC
- 28. Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell 2014;6:18. ArticlePubMedPMC
- 29. Suzuki H, Iwamoto H, Shimose S, Niizeki T, Shirono T, Noda Y, et al. Case report: exacerbation of varices following atezolizumab plus bevacizumab treatment of hepatocellular carcinoma: a case series and literature review. Front Oncol 2022;12:948293. ArticlePubMedPMC
References
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation
- Download Citation
- Close
- Related articles
-
- Liver resection in selective hepatocellular carcinoma with Vp3 or Vp4 portal vein tumor thrombosis improves prognosis
- Clinical characteristics and prognosis of Korean patients with hepatocellular carcinoma with respect to etiology
- Is multidisciplinary treatment effective for hepatocellular carcinoma with portal vein tumor thrombus?
- Long-term survival after CCRT and HAIC followed by ALPPS for hepatocellular carcinoma with portal vein invasion: a case report
- Concurrent transarterial radioembolization and combination atezolizumab/ bevacizumab treatment of infiltrative hepatocellular carcinoma with portal vein tumor thrombosis: a case report

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION


 Follow JLC on Twitter
Follow JLC on Twitter