Articles
- Page Path
- HOME > J Liver Cancer > Volume 23(2); 2023 > Article
-
Original Article
Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab -
Tae Hyun Kim1,2
 , Bo Hyun Kim1
, Bo Hyun Kim1 , Yu Ri Cho1
, Yu Ri Cho1 , Young-Hwan Koh1
, Young-Hwan Koh1 , Joong-Won Park1
, Joong-Won Park1
-
Journal of Liver Cancer 2023;23(2):330-340.
DOI: https://doi.org/10.17998/jlc.2023.04.14
Published online: May 16, 2023
1Center for Liver and Pancreatobiliary Cancer, National Cancer Center, Goyang, Korea
2Proton Therapy Center, National Cancer Center, Goyang, Korea
-
Corresponding author: Tae Hyun Kim, Proton Therapy Center, National Cancer Center, 323 Ilsan-ro, Ilsandong-gu, Goyang 10408, Korea
Tel. +82-31-920-1725, Fax. +82-31-920-0149 E-mail: k2onco@ncc.re.kr
© 2023 The Korean Liver Cancer Association.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,143 Views
- 124 Downloads
- 1 Citation
Abstract
-
Background/Aim
- Radiotherapy (RT) is an effective local treatment for hepatocellular carcinoma (HCC). However, whether additional RT is safe and effective in patients with advanced HCC receiving atezolizumab plus bevacizumab remains unclear. This retrospective cohort study aimed to evaluate the feasibility of additional RT in these patients.
-
Methods
- Between March and October 2021, we retrospectively analyzed seven patients with advanced HCC who received RT during treatment with atezolizumab plus bevacizumab. The median prescribed RT dose was 35 Gy (range, 33–66). Freedom from local progression (FFLP), progression-free survival (PFS), and overall survival (OS) after RT were analyzed.
-
Results
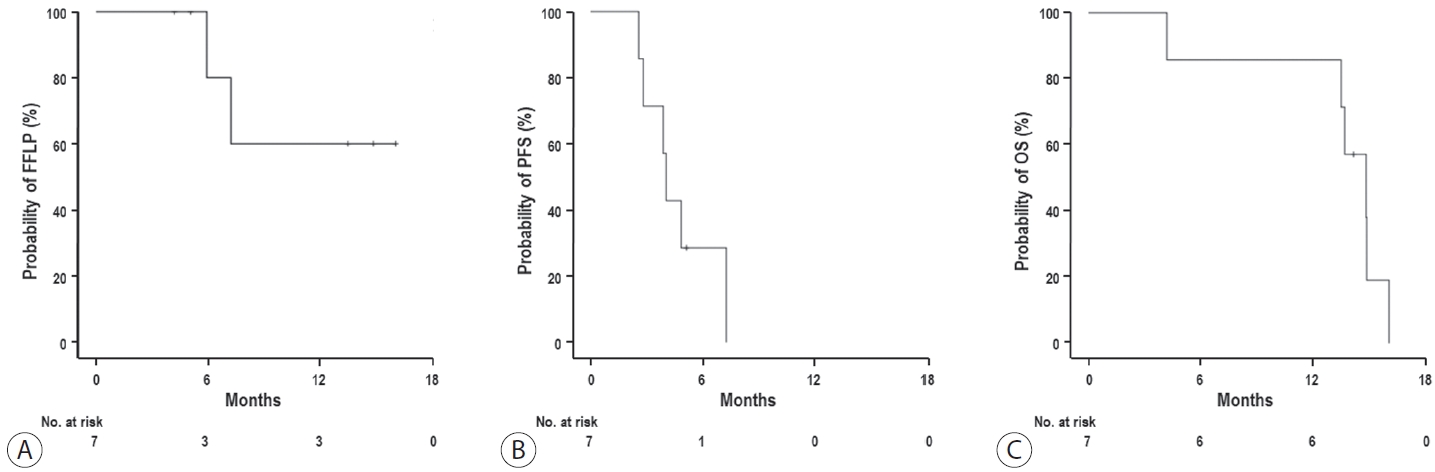
- The median follow-up duration after RT was 14.2 months (range, 10.0–18.6). Of the seven patients, disease progression was noted in six (85.7%), the sites of disease progression were local in two (28.6%), intrahepatic in four (57.1%), and extrahepatic in four (57.1%). The median time of FFLP was not reached, and PFS and OS times were 4.0 (95% confidence interval [CI], 3.6–4.5) and 14.8% (95% CI, 12.5–17.2) months, respectively. The 1-year FFLP, PFS, and OS rates were 60% (95% CI, 43.8–76.2), 0%, and 85.7% (95% CI, 75.9–95.5), respectively. Grade 3 or higher hematologic adverse events (AEs) were not observed, but grade 3 nonhematologic AEs unrelated to RT were observed in one patient.
-
Conclusions
- The addition of RT may be feasible in patients with advanced HCC treated with atezolizumab plus bevacizumab. However, further studies are required to validate these findings.
- Hepatocellular carcinoma (HCC) is the most common primary liver cancer and one of the leading causes of cancer-related deaths, primarily due to the presence of underlying chronic liver disease, late diagnosis, and frequent recurrence or progression after treatment.1,2 Treatment modalities for HCC include various local treatments, such as surgical resection, liver transplantation, radiofrequency ablation, percutaneous ethanol injection, transarterial chemoembolization, transarterial radioembolization, and radiotherapy (RT), as well as systemic treatments depending on the tumor stage, underlying liver function, and performance status.3-6 Although early stage HCC may be curable via surgical resection, liver transplantation, or ablation, most HCCs present as an advanced-stage and unresectable disease. Systemic treatments, such as those involving multikinase inhibitors (e.g., sorafenib and lenvatinib), have been used in patients with advanced and unresectable HCC despite their modest activity (i.e., modest improvement in survival [2–3 months] and low response rate [<5%]).7-9 The recent IMbrave150 trial showed significantly better survival outcomes with atezolizumab plus bevacizumab than with sorafenib.10,11 Nevertheless, prognosis remains poor in patients with advanced and unresectable HCC, even with systemic treatment, making it difficult to develop a treatment strategy without combining local and systemic treatments. Therefore, there is an unmet need for multidisciplinary treatments, that is, combinations of systemic and local treatment modalities, to improve treatment outcomes, and many studies have examined their efficacy and underlying mechanisms.12-16
- RT has emerged as a promising treatment modality for localized disease that is unsuitable for curative treatment or advanced disease with macroscopic vascular invasion and/or extrahepatic disease.17-31 Recent data have shown that radiation can cause immune-induced cell death and reprogram the tumor microenvironment against the immune avoidance mechanisms of cancer. Thus, the addition of RT to combined treatment with immunotherapeutic and anti-angiogenic agents (i.e., atezolizumab and bevacizumab) could enhance treatment outcomes by creating a synergistic effect.12-16,32-34 However, the safety and efficacy of additional RT in patients with advanced HCC undergoing treatment with atezolizumab plus bevacizumab remain unclear. Thus, we retrospectively evaluated patients with advanced HCC who received additional RT during treatment with atezolizumab plus bevacizumab and assessed the feasibility of additional RT in these patients.
INTRODUCTION
- 1. Patients
- The data of patients who received additional RT for advanced HCC during treatment with atezolizumab plus bevacizumab between March and October 2021 were retrospectively reviewed. Clinical and tumor stages were classified using the Barcelona Clinic Liver Cancer3 and American Joint Committee on Cancer35 staging classification, respectively. Data from the medical records of each patient, including age, sex, performance status, tumor size, clinical and tumor stage, baseline laboratory tests (alpha-fetoprotein, albumin, and bilirubin levels, etc.), treatment before RT, details of RT (prescribed radiation dose, irradiated site[s]), treatment after RT, and times and sites of disease progression, were collected. The collected data for each patient were assigned case numbers and anonymized. Data analyses were performed according to the relevant regulations, including good clinical practice and the Declaration of Helsinki guidelines. This study was approved by the Institutional Review Board of the National Cancer Center (NCC20230014), and the requirement for written informed consent was waived owing to the retrospective study design. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines were followed (Supplementary Table 1).
- 2. Treatment
- Standard doses of atezolizumab (1,200 mg per dose) and bevacizumab (15 mg/kg per dose) were administered to each patient every 3 weeks unless there was any specific reason for dose adjustment. The timing of RT was heterogeneous; however, patients were typically recommended the addition of RT during or at the time of treatment initiation with atezolizumab plus bevacizumab based on a multidisciplinary evaluation. RT with passive-scatter proton beam therapy or intensity-modulated RT was performed at the discretion of the physician and patient. The RT techniques, planning, and treatment procedures have been described in detail in our previous reports.21-26,28 Contrast-enhanced computed tomography (CT) was performed with each patient in the supine position, with immobilization and motion management considerations, including shallow respiration with abdominal compression or four-dimensional simulation. The gross tumor volume was defined as the tumor visualized on contrastenhanced CT images fused with dynamic CT and/or magnetic resonance images. The clinical target volume was defined as the sum of the gross tumor volume and internal target motion. Individualized planning target volume (PTV) margins of 0–7 mm from the clinical target volume were applied to compensate for respiratory motion and setup uncertainties and to ensure safe avoidance of organs at risk. Intensity-modulated RT and RT with passive-scatter proton beam therapy (Eclipse, version 13.7; Varian Medical Systems, Palo Alto, CA, USA) were performed using volumetric full or partial arcs of 6 MV X-ray and 230 MeV double-scattered proton beams (Proteus 235; Ion Beam Applications, Louvain-laNeuve, Belgium), respectively. Doses ranging from 33 to 66 Gy in 10 fractions were primarily prescribed depending on the dose-volume constraints of the organs at risk.21-26,28 During each treatment, all patients were instructed to fast for at least four hours before treatment, and the actual radiation was delivered after daily verification of the setup accuracy using either digital orthogonal X-rays or kilovoltage cone-beam CT.
- 3. Assessments and statistical analysis
- Clinical, laboratory (including alpha-fetoprotein levels), and radiological examinations (including dynamic liver CT and magnetic resonance imaging) were performed every 2–3 months for the first 2 years and every 6 months thereafter. The albumin-bilirubin (ALBI) score was calculated using the formula: ALBI score = (log10 bilirubin [µmol/L] × 0.66) + (albumin [g/L] × -0.085), and the ALBI grades were classified based on the specific cutoff values: grade 1, ≤-2.60; grade 2, >-2.60 and ≥-1.39; and grade 3, >-1.39.36 Tumor response and disease progression were assessed based on the Response Evaluation Criteria in Solid Tumors (version 1.1)37 and RT-related adverse events (AEs) were assessed according to the Common Terminology Criteria for AEs (version 4.03). Disease progression was classified according to the site of growth or new tumors as follows: local progression within the PTV, intrahepatic progression within the liver outside the PTV, and extrahepatic progression outside the liver, such as in the regional or non-regional lymph nodes and distant organs. The time of freedom from local progression (FFLP), progression-free survival (PFS), and overall survival (OS) were determined from the commencement date of RT to the date of local progression, disease progression or death, and death from any cause or the last follow-up, respectively. The probability of survival was estimated using the Kaplan–Meier method, and all statistical analyses were performed using the STATA software (version 14.0; StataCorp, College Station, TX, USA).
METHODS
- Between March and October 2021, seven patients received additional RT during combined treatment with atezolizumab and bevacizumab at our institute. Of the seven patients, five received RT after one cycle of atezolizumab + bevacizumab, while two received RT after three and seven cycles of atezolizumab + bevacizumab, respectively. A median of seven cycles (range, 3–9) of atezolizumab plus bevacizumab treatment were continued after RT. The patient and pretreatment characteristics are summarized in Table 1. The target sites of RT were tumor thrombosis of the major vessels (n=3) and metastatic lesions (peritoneal seeding [n=2], bone [n=1], and adrenal glands and para-aortic lymph nodes [n=1]). The median prescribed dose of RT was 35 Gy (range, 33–66) in 10 and 5 fractions/week. The targeted lesion(s) and overall tumor response in the patients were as follows: complete response in one (14.3%) and one (14.3%), partial response in four (57.1%) and one (14.3%), stable disease in two (28.6%) and four (57.4%), and progressive disease in 0 (0%) and one (14.3%), respectively (Fig. 1). The median duration of follow-up for all patients after RT was 14.2 months (range, 10.0–18.6).
- At the time of the analysis, one patient was alive, while five had died due to disease progression and one had died of an unconfirmed cause due to follow-up loss from 5 months after RT. Disease progression occurred in six of the seven (85.7%) patients, with the initial sites being local in one (14.3%), intrahepatic in three (42.9%), and extrahepatic in four (57.1%) (Table 2). The sites of disease progression were local in two (28.6%), intrahepatic in four (57.1%), and extrahepatic in four (57.1%) patients (Table 2). Two patients experienced local progression at 5.9 and 7.2 months after RT, respectively, while disease progression was observed in six patients at a median of 4.0 months (range, 2.7–4.5) after RT (Table 2). After confirmation of disease progression, five patients received one or a combination of systemic and local treatments (i.e., transarterial chemoembolization, RT, atezolizumab, lenvatinib, nivolumab, etc.), and two patients received the best supportive care, considering their performance status and willingness (Table 2). The median time of FFLP was not reached, and the PFS and OS times were 4.0 (95% confidence interval [CI], 3.6–4.5), and 14.8 months (95% CI, 12.5–17.2), respectively. The 1-year FFLP, PFS, and OS rates were 60% (95% CI, 43.8–76.2), 0%, and 85.7% (95% CI, 75.9–95.5), respectively (Fig. 2).
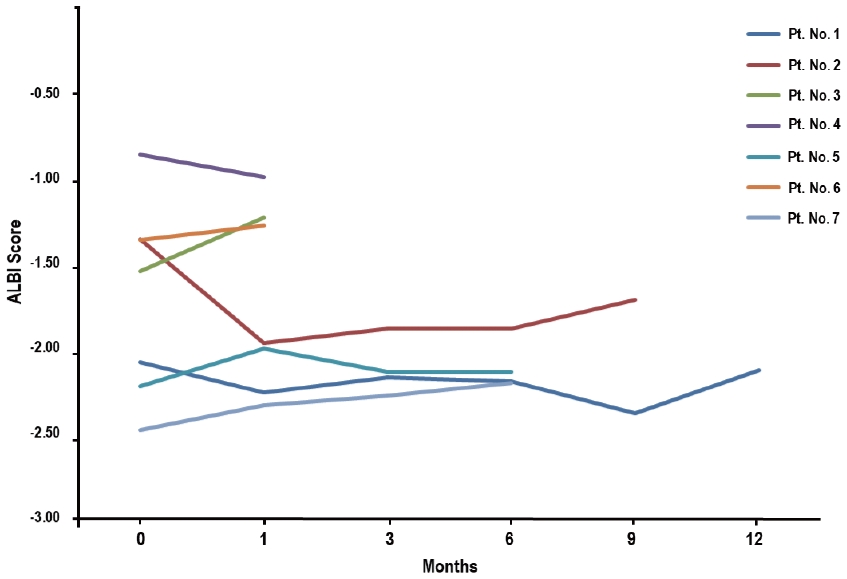
- AEs during and after RT are summarized in Table 3. Among the seven patients who received additional RT during treatment with atezolizumab plus bevacizumab, all experienced hematologic AEs, with three (42.9%) experiencing grade 1 and four (47.1%) experiencing grade 2, but none experienced ≥ grade 3 hematologic AEs (Table 3). Elevated alanine aminotransferase, hypoalbuminemia, and hyperbilirubinemia levels without evidence of tumor progression were observed in one patient (14.3%), all of which were of grade 1. Of the seven patients, four (57.1%) showed no change in their Child-Pugh scores, two (28.6%) showed a 1-point decrease, and one (14.3%) showed a 1-point increase. The changes in the ALBI scores of each patient are shown in Fig. 3. Of the seven patients, five (71.4%) showed no change in the ALBI grade, one (14.3%) showed a 1-grade decrease, and one (14.3%) showed a 1-grade increase. The median change in the ALBI score was -0.04 (range, -0.61 to 0.310). Non-hematologic AEs, i.e., grade 1, fever; grade 2, epistaxis; and grade 3, gastric varix bleeding that was unrelated to the direct impact of RT as the site of bleeding was outside the RT field, were observed in one patient (14.3%), but non-hematologic AEs of grade ≥4 were not observed (Table 3).
RESULTS
- Combination of atezolizumab and bevacizumab has emerged as the first-line treatment for patients with unresectable and extrahepatic HCC, showing superior PFS and OS rates compared to those in targeted systemic therapies, such as sorafenib.10,11 The use of local treatments, including RT, in patients with HCC with macroscopic vascular invasion and extrahepatic disease can improve tumor control and the quality of life of patients by decreasing tumor-related symptoms and morbidities.21,24,27,29,30 The combination of RT and systemic treatments, such as atezolizumab plus bevacizumab, is theoretically expected to have synergistic effects,14-16 however, it is unclear whether the addition of RT to atezolizumab plus bevacizumab treatment in patients with advanced HCC is a potentially effective treatment option because of the lack of relevant studies.12,13 Although the population in the present study was small, it showed a promising objective response rate of 71.4% and 1-year FFLP rate of 60% in targeted lesion(s) treated with RT. Furthermore, the overall objective response rate was 28.6% and disease control rate was 85.7%, which were slightly higher and comparable to those of sorafenib and atezolizumab plus bevacizumab treatments (11.9 and 27.3%, and 55.3 and 73.6%, respectively) in the IMbrave150 trial.10,11 In this trial, only patients with a Child-Pugh score of ≤6 (5 [72%] and 6 [28%]) were enrolled and 77% of the patients had advanced stage disease, such as macrovascular invasion and/or extrahepatic disease. Sorafenib and atezolizumab plus bevacizumab treatments showed median OS times of 13.4 and 19.2 months and 1-year OS rates of 54.6% and 67.2%, respectively. In the present study, 57.1% patients had a Child-Pugh score of ≥7 and 100% of patients had advanced stage disease. Additional RT in patients treated with atezolizumab plus bevacizumab showed a median OS time of 14.8 months and 1-year OS rate of 85.7%. Considering that a high proportion of patients in the present study had poor liver function and/or advanced-stage disease, the survival outcomes of additional RT in patients with advanced HCC treated with atezolizumab plus bevacizumab are promising.
- When considering additional RT in patients with advanced HCC treated with atezolizumab plus bevacizumab, it is important to consider its safety and efficacy. In the present study, all-cause AEs of any grade occurred in all patients; however, ≥grade 3 AEs occurred in only one patient (14.3%). In the IMbrave150 trial,11,13 atezolizumab plus bevacizumab treatment resulted in an any-grade AEs rate of 98% and ≥grade 3 AEs rate of 63%. In addition, there are concerns regarding gastrointestinal AEs, such as bleeding, when combining RT with anti-angiogenic agents, such as bevacizumab; thus, discontinuation of anti-angiogenic agents is sometimes considered during RT.13 In the present study, although bevacizumab was not discontinued during RT, grade 3 AEs occurred in one patient (14.3%) that were unrelated to RT because the bleeding site was outside the RT field. These results suggest that additional RT may be safe in patients with HCC treated with atezolizumab plus bevacizumab. However, the present study was a retrospective analysis that included a small study population (n=7). Retrospective studies are generally likely to underestimate the incidence of AEs because of recall bias and incomplete medical records. In addition, the efficacy and toxicity of the sequence of RT and atezolizumab plus bevacizumab remain unclear; however, in this study, RT was performed immediately after one cycle of atezolizumab plus bevacizumab treatment in most patients (71.4%) on the basis of a multidisciplinary evaluation considering the potential synergies between RT and atezolizumab plus bevacizumab. Further large-scale prospective studies are needed to confirm the safety and efficacy of additional RT in patients with advanced HCC receiving atezolizumab plus bevacizumab treatment.
- In conclusion, RT may be a feasible and potentially effective treatment option in patients with advanced HCC treated with atezolizumab plus bevacizumab, and the addition of RT can be well-tolerated. Further studies are required to validate these findings and assess the safety and efficacy of this treatment approach.
DISCUSSION
-
Conflict of Interest
The authors have no conflicts of interest to disclose.
-
Ethics Statement
This study was approved by the Institutional Review Board of the National Cancer Center (NCC) (IRB No. NCC20230014), and the requirement for written informed consent was waived because of the retrospective study design.
-
Funding Statement
This research was funded by a National Cancer Center Grant (NCC2110351) and supported by the Korean Liver Cancer Association (2022). The funding source had no role in the study design, collection, analysis, or interpretation of the data.
-
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical regulations.
-
Author Contribution
Conceptualization: THK
Data curation: THK, BHK, YC, YK, JP
Formal analysis: THK
Funding acquisition: THK
Methodology: THK, BHK, YC, YK, JP
Project administration: THK
Writing original draft: THK
Writing review & editing: THK, BHK, YC, YK, JP
Approval of final manuscript: all authors
Article information
Supplementary Material

Pt, patient; ECOG PS, Eastern Cooperative Oncology Group performance status; CP, Child-Pugh; ALBI, albumin-bilirubin; AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer; Tx, treatment; RT, radiotherapy; Dz, disease; Prev, previous; M, male; TACE, transarterial chemoemboization; SR, surgical resection; P, peritoneal; Ate, atezolizumab; Beva, bevacizumab; BM, both main portal vein; TT, tumor thrombosis; IHD, intrahepatic disease; IVC, inferior vena cava tumor thrombosis; EHD, extrahepatic disease; Adr Gl, adrenal gland; PAN, para-aortic lymph node.
Pt, patient; RT, radiotherapy; TD, total radiation dose; Subseq, subsequent; Tx, treatment; TLR, targeted lesion(s) response; OR, overall response; PD, progressive disease; TI, time interval; LP, local progression; PFS, progression free survival; OS, overall survival; PBT, proton beam therapy; Ate, atezolizumab; Beva, bevacizumab; CR, complete response; IHD, intrahepatic disease other than the targeted lesion(s); EHD, extrahepatic disease other than the targeted lesion(s); TACE, transarterial chemoemboization; Lenva, lenvatinib; DWD, death with disease; PR, partial response; SD, stable disease; IMRT, intensity modulated radiotherapy; Nivo, nivolumab; GP, gemcitabine and cisplatin; AWD, alive with disease.
| CTCAE grade |
All patients (n=7) |
||||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||
| Hematologic AEs | 3 (42.9) | 4 (57.1) | 0 (0.0) | 0 (0.0) | |
| WBC increase | 1 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| WBC decrease | 3 (42.9) | 3 (42.9) | 0 (0.0) | 0 (0.0) | |
| PLT decrease | 1 (14.3) | 2 (28.6) | 0 (0.0) | 0 (0.0) | |
| ALT/AST increase | 1 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Albumin decrease | 1 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Bilirubin increase | 1 (14.3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Non-hematologic AEs | 1 (14.3) | 1 (14.3) | 1 (14.3) | 0 (0.0) | |
| Fever | 1 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Pain | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Dermatitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Radiation pneumonitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Bleeding | 0 (0.0) | 1 (14.3)* | 1 (14.3)† | 0 (0.0) | |
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209−249.ArticlePubMedPDF
- 2. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int 2015;35:2155−2166.ArticlePubMedPMCPDF
- 3. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182−236.ArticlePubMed
- 4. Korean Liver Cancer Association, National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver 2019;13:227−299.ArticlePubMedPMC
- 5. Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer 2021;10:181−223.ArticlePubMedPMCPDF
- 6. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723−750.ArticlePubMedPDF
- 7. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378−390.ArticlePubMed
- 8. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25−34.ArticlePubMed
- 9. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 noninferiority trial. Lancet 2018;391:1163−1173.ArticlePubMed
- 10. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 2022;76:862−873.ArticlePubMed
- 11. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894−1905.ArticlePubMed
- 12. Manzar GS, De BS, Abana CO, Lee SS, Javle M, Kaseb AO, et al. Outcomes and toxicities of modern combined modality therapy with atezolizumab plus bevacizumab and radiation therapy for hepatocellular carcinoma. Cancers (Basel) 2022;14:1901. ArticlePubMedPMC
- 13. Chiang CL, Chan ACY, Chiu KWH, Kong FS. Combined stereotactic body radiotherapy and checkpoint inhibition in unresectable hepatocellular carcinoma: a potential synergistic treatment strategy. Front Oncol 2019;9:1157. ArticlePubMedPMC
- 14. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687−695.ArticlePubMedPMC
- 15. Kim KJ, Kim JH, Lee SJ, Lee EJ, Shin EC, Seong J. Radiation improves antitumor effect of immune checkpoint inhibitor in murine hepatocellular carcinoma model. Oncotarget 2017;8:41242−41255.ArticlePubMedPMC
- 16. Young KH, Baird JR, Savage T, Cottam B, Friedman D, Bambina S, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One 2016;11:e0157164.ArticlePubMedPMC
- 17. Fukuda K, Okumura T, Abei M, Fukumitsu N, Ishige K, Mizumoto M, et al. Long-term outcomes of proton beam therapy in patients with previously untreated hepatocellular carcinoma. Cancer Sci 2017;108:497−503.ArticlePubMedPMCPDF
- 18. Fukumitsu N, Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Abei M, et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2009;74:831−836.ArticlePubMed
- 19. Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blaszkowsky LS, et al. Multi-institutional phase ii study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2016;34:460−468.ArticlePubMedPMC
- 20. Kawashima M, Furuse J, Nishio T, Konishi M, Ishii H, Kinoshita T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol 2005;23:1839−1846.ArticlePubMed
- 21. Kim DY, Park JW, Kim TH, Kim BH, Moon SH, Kim SS, et al. Riskadapted simultaneous integrated boost-proton beam therapy (SIB-PBT) for advanced hepatocellular carcinoma with tumour vascular thrombosis. Radiother Oncol 2017;122:122−129.ArticlePubMed
- 22. Kim TH, Kim BH, Park JW, Cho YR, Koh YH, Chun JW, et al. Proton beam therapy for treatment-naïve hepatocellular carcinoma and prognostic significance of albumin-bilirubin (ALBI) grade. Cancers (Basel) 2022;14:4445. ArticlePubMedPMC
- 23. Kim TH, Koh YH, Kim BH, Kim MJ, Lee JH, Park B, et al. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: a randomized phase III trial. J Hepatol 2021;74:603−612.ArticlePubMed
- 24. Kim TH, Park JW, Kim BH, Kim H, Moon SH, Kim SS, et al. Does risk-adapted proton beam therapy have a role as a complementary or alternative therapeutic option for hepatocellular carcinoma? Cancers (Basel) 2019;11:230. ArticlePubMedPMC
- 25. Kim TH, Park JW, Kim BH, Oh ES, Youn SH, Moon SH, et al. Phase II study of hypofractionated proton beam therapy for hepatocellular carcinoma. Front Oncol 2020;10:542. ArticlePubMedPMC
- 26. Kim TH, Park JW, Kim YJ, Kim BH, Woo SM, Moon SH, et al. Phase I dose-escalation study of proton beam therapy for inoperable hepatocellular carcinoma. Cancer Res Treat 2015;47:34−45.ArticlePubMedPMCPDF
- 27. Kim Y, Park HC, Yoon SM, Kim TH, Lee J, Choi J, et al. Prognostic group stratification and nomogram for predicting overall survival in patients who received radiotherapy for abdominal lymph node metastasis from hepatocellular carcinoma: a multi-institutional retrospective study (KROG 15-02). Oncotarget 2017;8:94450−94461.ArticlePubMedPMC
- 28. Kim TH, Park JW, Kim YJ, Kim BH, Woo SM, Moon SH, et al. Simultaneous integrated boost-intensity modulated radiation therapy for inoperable hepatocellular carcinoma. Strahlenther Onkol 2014;190:882−890.ArticlePubMedPDF
- 29. Jung J, Yoon SM, Park HC, Nam TK, Seong J, Chie EK, et al. Radiotherapy for adrenal metastasis from hepatocellular carcinoma: a multi-institutional retrospective study (KROG 13-05). PLoS One 2016;11:e0152642.ArticlePubMedPMC
- 30. Lee SU, Park JW, Kim TH, Kim YJ, Woo SM, Koh YH, et al. Effectiveness and safety of proton beam therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Strahlenther Onkol 2014;190:806−814.ArticlePubMedPDF
- 31. Cho Y, Kim BH, Kim TH, Koh YH, Park JW. Sorafenib combined with radiation therapy for advanced hepatocellular carcinoma with portal and hepatic vein invasion extending to the inferior vena cava: a complete response case according to modified RECIST criteria. J Liver Cancer 2022;22:63−68.ArticlePubMedPMCPDF
- 32. Cho Y, Kim BH, Kim TH, Koh YH, Park JW. A case of successful surgical treatment for peritoneal seeding of hepatocellular carcinoma after radiotherapy and atezolizumab plus bevacizumab combination treatment. J Liver Cancer 2023;23:206−212.ArticlePubMedPMCPDF
- 33. Lee A, Lee J, Yang H, Sung SY, Jeon CH, Kim SH, et al. Multidisciplinary treatment with immune checkpoint inhibitors for advanced stage hepatocellular carcinoma. J Liver Cancer 2022;22:75−83.ArticlePubMedPMCPDF
- 34. Kim YT, Kim J, Seong J. Favorable response of hepatocellular carcinoma with portal vein tumor thrombosis after radiotherapy combined with atezolizumab plus bevacizumab. J Liver Cancer 2023;23:225−229.ArticlePubMedPMCPDF
- 35. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual, 8th ed. Berlin, Springer. 2017.
- 36. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550−558.ArticlePubMedPMC
- 37. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228−247.ArticlePubMed
References
Figure & Data
References
Citations

- Letter regarding “Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab”
Sun Hyun Bae, Hee Chul Park
Journal of Liver Cancer.2023; 23(2): 402. CrossRef
 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation
- Download Citation
- Close
- Related articles
-
- Additional nodules detected using EOB-MRI in patients with resectable single hepatocellular carcinoma: an implication for active treatment strategy
- Complications of immunotherapy in advanced hepatocellular carcinoma
- A multidisciplinary approach with immunotherapies for advanced hepatocellular carcinoma
- Letter regarding “Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab”
- How to optimize the treatment strategy for advanced-stage hepatocellular carcinoma with macrovascular invasion

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION


 Follow JLC on Twitter
Follow JLC on Twitter