Articles
- Page Path
- HOME > J Liver Cancer > Volume 22(2); 2022 > Article
-
Original Article
Clinical characteristics and prognosis of Korean patients with hepatocellular carcinoma with respect to etiology -
Wonjoon Jang1*
 , Hye Won Lee1,2,3*
, Hye Won Lee1,2,3* , Jae Seung Lee1,2,3
, Jae Seung Lee1,2,3 , Beom Kyung Kim1,2,3
, Beom Kyung Kim1,2,3 , Seung Up Kim1,2,3
, Seung Up Kim1,2,3 , Jun Yong Park1,2,3
, Jun Yong Park1,2,3 , Sang Hoon Ahn1,2,3
, Sang Hoon Ahn1,2,3 , Do Young Kim1,2,3
, Do Young Kim1,2,3
-
Journal of Liver Cancer 2022;22(2):158-166.
DOI: https://doi.org/10.17998/jlc.2022.09.18
Published online: September 27, 2022
1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
2Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea
3Yonsei Liver Center, Severance Hospital, Seoul, Korea
-
Corresponding author: Do Young Kim Department of Internal Medicine, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea
Tel. +82-2-2228-1992, Fax. +82-2-393-6884 E-mail: dyk1025@yuhs.ac - *These two authors contributed equally to this work.
Copyright © 2022 The Korean Liver Cancer Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 3,140 Views
- 72 Downloads
- 7 Citations
Abstract
-
Background/Aim
- The profile of patients with hepatocellular carcinoma (HCC) has changed globally; the role of etiology in predicting prognosis of HCC patients remains unclear. We aimed to analyze the characteristics and prognosis of Korean patients with HCC according to disease etiology.
-
Methods
- This retrospective observational study included patients diagnosed with HCC between 2010 and 2014 in a single center in Korea. Patients with HCC aged <19 years old, had coinfection with other viral hepatitis, had missing follow-up data, were Barcelona Clinic Liver Cancer stage D, or died before 1 month were excluded.
-
Results
- A total of 1,595 patients with HCC were analyzed; they were classified into the hepatitis B virus (HBV) group (1,183 [74.2%]), hepatitis C virus (HCV) group (146 [9.2%]), and non-B non-C (NBNC) group (266 [16.7%]). The median overall survival of all patients was 74 months. The survival rates at 1, 3, and 5 years were 78.8%, 62.0% and 54.9% in the HBV group; 86.0%, 64.0%, and 48.6% in the HCV group; and 78.4%, 56.5%, and 45.9% in the NBNC group, respectively. NBNC-HCC has a poorer prognosis than other causes of HCC. Survival was significantly longer in the HBV group with early-stage HCC than in the NBNC group. Furthermore, survival was shorter in patients with early-stage HCC and diabetes mellitus (DM) than in those without DM.
-
Conclusions
- The etiology of HCC affected clinical characteristics and prognosis to some extent. NBNC-HCC patients showed shorter overall survival than viral-related HCC patients. Additionally, the presence of DM is an additional important prognostic factor in patients with early-stage HCC.
- Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, the sixth most common cancer worldwide, and the fourth leading cause of cancer-related mortality.1-3 HCC is mainly associated with chronic viral hepatitis including hepatitis B virus (HBV) or hepatitis C virus (HCV).4 In South Korea, HBV infection is 65-75% of HCC cases diagnosed, while HCV infection is associated with 8.6-13.2% of cases.5,6 Recently, non-B non-C (NBNC) HCC has gradually increased and been reported to be 15-18% in Korea.6
- Various factors influence the development of HCC through different mechanisms.7 HBV is a DNA-based virus that can be incorporated into the host genome. Random insertions cause chromosomal instability that drives carcinogenesis.8,9 HCV is an RNA virus that does not integrate into host DNA. Due to the absence of reverse transcription activity of the HCV RNA virus, its viral genome does not integrate into the genome of the infected cell. HCV causes HCC through an indirect pathway by causing chronic inflammation, cell death, proliferation and cirrhosis.8 In addition, tumorigenesis is associated with non-viral risk factors such as fatty liver disease or heavy alcohol consumption.10 NBNC population constitutes a substantial proportion of patients with HCC and has become the main cause of liver transplantation.
- Most HCC cases develop in patients with cirrhosis. Generally, genetic and epigenetic mechanisms play an important role in the malignant transformation of dysplastic nodules.11 Given the different etiologies and characteristics of HCC, the prognosis may vary between HBV, HCV, and NBNC patients. However, the earlier results are somewhat controversial. In a recent study, HBV-related patients showed favorable survival compared with other etiologies.12 Some studies found significant survival differences between groups with different risk factors for HCC,13-18 while others did not.19-23 Furthermore, most studies only compared virus-infected groups (HBV vs. HCV) or combined both viral groups when compared to patients with NBNC.
- However, the profiles of patients with HCC are changing. Remarkable treatment advances include immunotherapy, tyrosine kinase inhibitor, and transarterial radioembolization, which increase survival and quality of life but remain under evaluation. The NBNC-HCC population is increasing and currently outnumbers the HCV-HCC population in some regions.24 Therefore, it is crucial to explore the role of etiology in HCC prognosis. In this study, we analyzed the characteristics of patients with HCC and their prognosis according to the etiology of the disease in Korea.
INTRODUCTION
- 1. Study population
- Between 2010 and 2014, 1,900 patients who were first diagnosed with HCC at Severance Hospital, Yonsei University College of Medicine, South Korea, were enrolled. HCC was diagnosed histologically or radiologically according to the guidelines of the Korean Liver Cancer Association, American Association for the Study of Liver Disease or the European Association for the Study of the Liver.23 Patients aged <19 years, those who had coinfection with other viral hepatitis, those who had missing follow-up data, or those who died before 1 month were excluded (Supplementary Fig. 1). Additionally, patients with Barcelona Clinic Liver Cancer (BCLC) stage D were excluded.
- The patients were divided according to the etiology of the disease into three groups (HBV, HCV, and NBNC). Patients with HBsAg-positivity for ≥6 months, previous history of chronic HBV infection, and anti-HCV antibody-negative sera were assigned to the HBV group, those with anti-HCV antibody positive and HBsAg-negative sera were assigned to the HCV group. The remaining patients negative for HBV and HCV were assigned to the NBNC group; therefore, this was a heterogeneous group and included those whose HCC was attributable to alcoholic liver disease.
- This retrospective observational study followed the STROBE guidelines (Supplementary Table 1). This study adhered to the ethical guidelines of the Declaration of Helsinki (1975). This study was approved by the Severance Hospital Institutional Review Board (IRB No. 4-2020-1081).
- 2. Variables and outcomes
- All clinical, serological, and histological data were obtained from electronic medical records. Clinical variables included age, hypertension, type 2 diabetes mellitus (DM), sex, smoking, alcohol consumption (social drinking), BCLC stage, Child-Pugh score, and initial treatment (transarterial chemoembolization, transarterial radioembolization, radiofrequency ablation, cryoablation, systemic or intra-arterial chemotherapy, radiation, concurrent chemoradiation, surgical resection, etc.). Computed tomography and magnetic resonance imaging were used to explore tumor size, macrovascular invasion (MVI), and extrahepatic metastasis. We recorded the following serologic variables: albumin, total bilirubin, alpha-fetoprotein (AFP), prothrombin induced by the absence of vitamin K or antagonist-II (PIVKA-II), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and prothrombin time expressed as the international normalized ratio. All data were obtained using routine methods. The primary outcome was overall survival (OS), determined from the date of initial diagnosis to death or the last follow-up date.
- 3. Statistical methods
- Pearson’s chi-square test was used to compare baseline categorical variables. Analysis of variance or the Kruskal–Wallis test was used to compare continuous variables. OS was calculated using the Kaplan–Meier method. The association between each baseline variable and survival was tested using univariate analysis (log-rank test). Significant variables (P<0.05) in the univariate analysis were included in the multivariate analysis. All statistical analyses were performed with SPSS software ver. 22.0 (SPSS Inc, Chicago, IL, USA). Two-sided P<0.05 were considered statistically significant.
METHODS
- 1. Baseline characteristics of the study population
- The baseline characteristics of the study population are summarized in Table 1. Finally, 1,595 patients with HCC were analyzed. Of the 1,595 patients, 1,183 (74.2%) were in the HBV group, 146 (9.2%) were in the HCV group, and 266 (16.7%) were in the NBNC group. The HCV group had the highest proportion of female patients. The NBNC group had more elderly patients and metabolic comorbidities, including hypertension or type 2 DM than the viral hepatitis group. The HCV group had the lowest serum albumin level (3.5 g/dL vs. 3.8 g/dL and 3.7 g/dL, P<0.001) and the highest AST level (60.0 U/L vs. 45.0 U/L and 39.5 U/L, P<0.001) compared to the HBV and NBNC groups. The HBV group had the highest ALT level (36.0 U/L) compared to the HCV group (32.0 U/L) and the NBNC group (26.0 U/L) (P<0.001).
- The NBNC group had the largest mean tumor size (3.6 cm vs. 3.5 cm in the HBV group and 3.3 cm in the HCV group, P=0.005). MVI was the least common in the HCV group (19.9% vs. 30.0% in the HBV group and 24.8% in the NBNC group, P=0.015). However, the extrahepatic metastasis rate did not differ between the three groups. The proportion of BCLC stage C patients was lower in the HCV group (23.3%) than in the HBV (35.2%) and NBNC groups (33.1%). The most common initial treatment in all groups was transarterial chemoembolization, followed by surgical resection.
- 2. OS according to the etiology
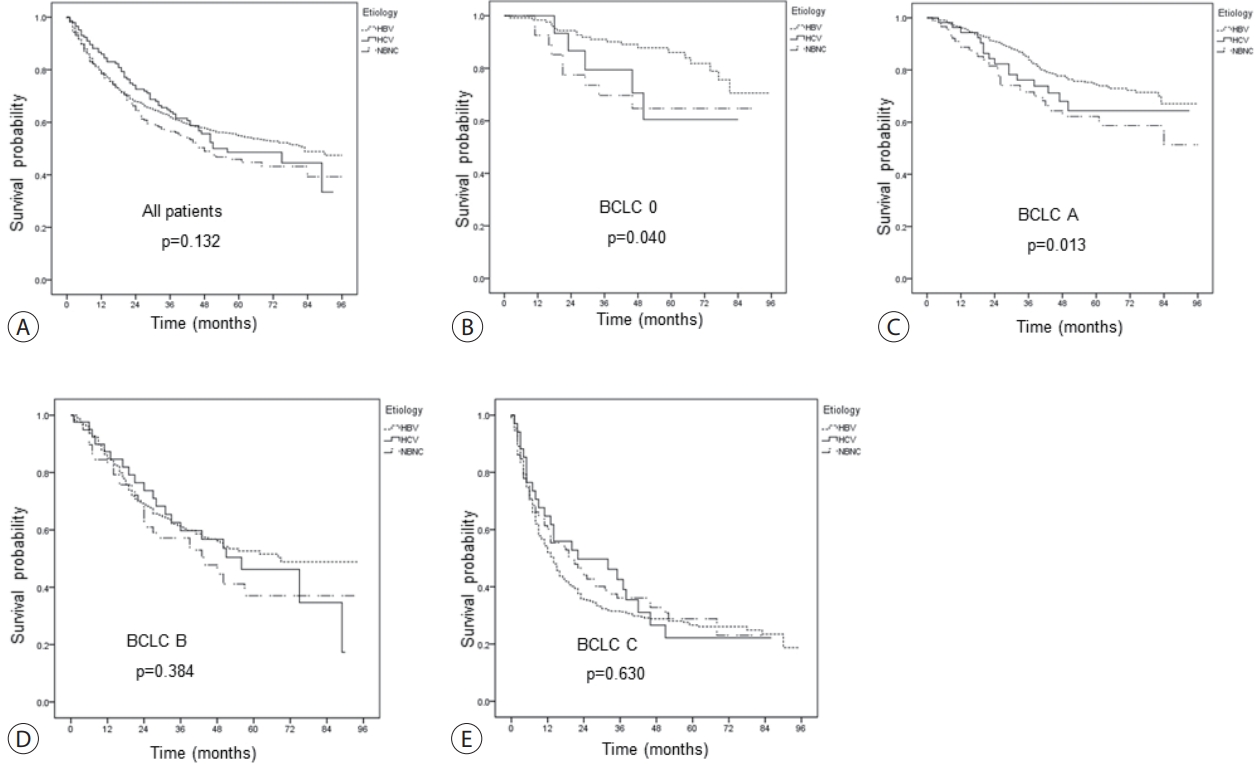
- The median OS of all patients was 74 months. The survival rates at 1, 3, and 5 years were 78.8%, 62%, and 54.9% in the HBV group; 86.0%, 64.0%, and 48.6% in the HCV group; and 78.4%, 56.5%, and 45.9% in the NBNC group, respectively, and were comparable between the groups (Fig. 1A). However, in the BCLC stage 0 or A subgroup, HBV patients had significantly longer OS than NBNC patients (Fig. 1B-E).
- 3. Risk factors for mortality in patients with HCC
- The log-rank test was used to identify factors that predicting prognosis. Univariate analysis identified the following factors: NBNC etiology, albumin, total bilirubin, prothrombin time, AST, tumor size, MVI, extrahepatic metastasis, AFP, and PIVKA-II (Table 2). Factors with P<0.05 were included in the multivariate analysis, which identified the following significant risk factors for mortality: NBNC etiology (hazard ratio [HR], 1.245; 95% confidence interval [CI], 1.020-1.518; P=0.031), low albumin level (HR, 0.629; 95% CI, 0.538-0.734; P<0.001), high prothrombin time (HR <2.314; 95% CI, 1.280-4.182; P=0.005), MVI (HR, 2.682; 95% CI, 2.278-3.158; P<0.001), extrahepatic metastasis status (HR, 2.882; 95% CI, 2.233-3.720; P<0.001), high AFP (HR, 1.000; 95% CI, 1.000-1.000; P<0.001), and PIVKA-II (HR, 1.000; 95% CI, 1.000-1.000; P<0.001) levels (Table 2). We also analyzed the risk factors for mortality in each stage of BCLC (Supplementary Tables 2-5).
- 4. The effects of DM on the risk of mortality
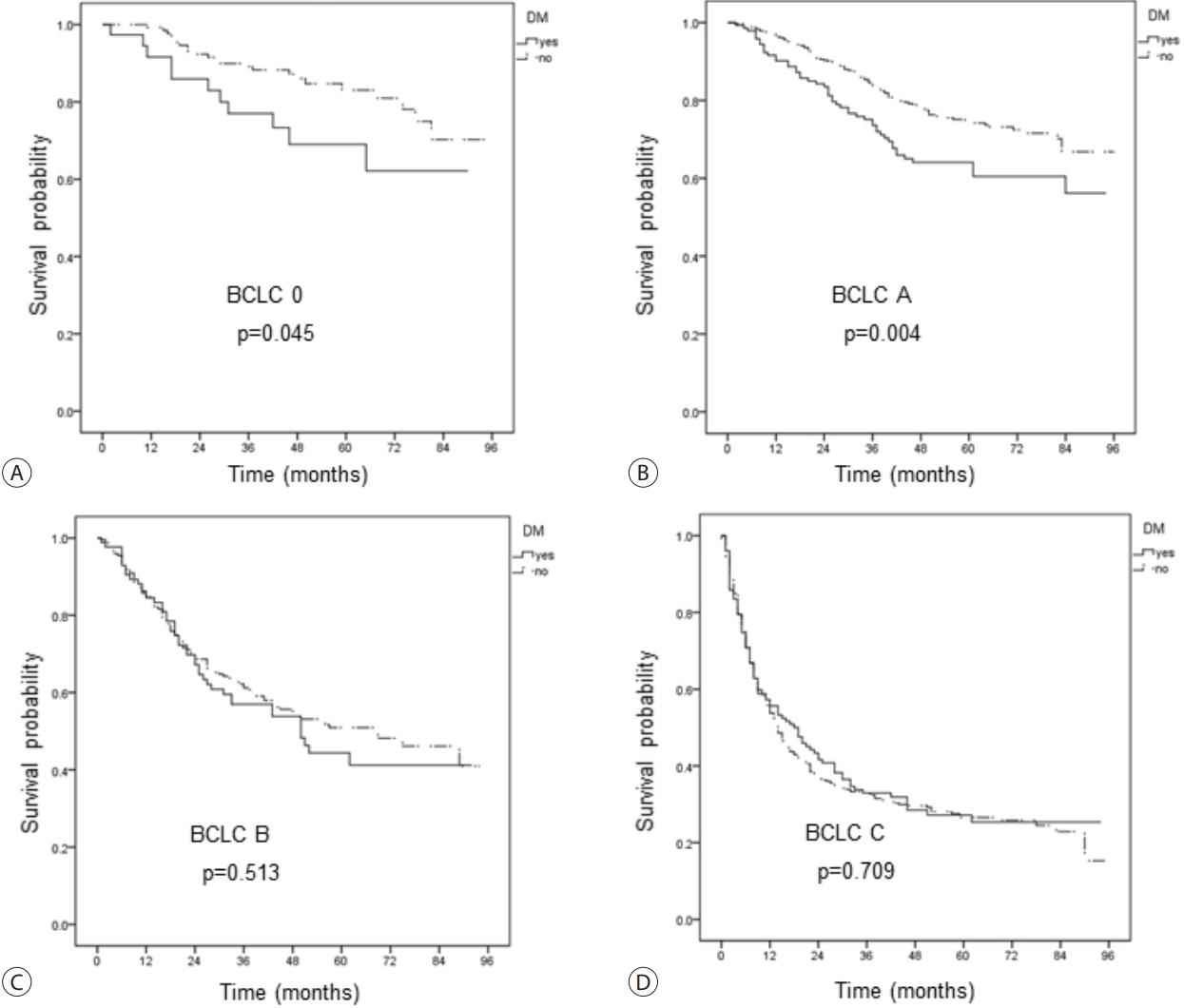
- We explored the effect of type 2 DM on OS (Fig. 2A). Overall, DM was a risk factor for mortality in patients with early-stage HCC, including BCLC stage 0 or A, but not B or C (Fig. 2B-D). Similar results were obtained when the HBV and NBNC sub-groups were analyzed (Table 3). DM increased the risk of mortality in HCC patients with BCLC 0 (HR, 2.06; 95% CI, 1.00-4.22; P=0.049) and BCLC A (HR, 1.65; 95% CI, 1.17-2.31; P=0.004) in overall population. In HBV-HCC and NBNC-HCC only, DM similarly increased the risk of mortality in HCC patients with BCLC 0 (HR, 2.97; 95% CI, 1.40-6.30; P=0.005) and BCLC A (HR, 1.78; 95% CI, 1.25-2.53; P=0.002) in overall population.
RESULTS
- We explored whether the etiology of HCC affected disease characteristics and prognosis. As previously reported, we found that the mean age of HCV and NBNC patients was approximately 10 years older than that of HBV patients.14,16,21 The NBNC group had the lowest serum AST and ALT levels. Although liver enzyme levels differed between the groups, the Child–Pugh score, liver function, and prognosis did not show differences. The tumor size was the largest in the NBNC group. The frequency of MVI was the highest in the HBV group. When the groups were evaluated in terms of extrahepatic metastasis, BCLC stage distribution, and initial treatment, the aggressiveness of HCC did not show differences between the groups. The HBV group had significantly longer OS than the NBNC group in HCC patients with early stage. Therefore, we stratified the patients according to BCLC stage and found that HBV patients with BCLC stages 0 and A (but not B or C) survived significantly longer than those with NBNC. However, this should be interpreted with caution, as the proportion of patients with DM in the NBNC group was more than twice that of the HBV group.
- We also speculate that DM may affect survival differently according to the HCC stage. The results of the univariate analysis, shown in Table 3 and Fig. 2, support this conclusion. DM was a significant independent predictor of poorer survival in patients with BCLC stages 0 and A, but not B or C. Others have reported that DM in patients with HCC is a prognostic factor. One study from Taiwan found that DM was a crucial predictor of survival in patients with early-stage HCC (BCLC 0 and A).25 DM can enhance or reduce survival depending on the BCLC stage or treatment.25-27 We did not consider other confounders (metabolic syndrome or insulin resistance) that could contribute to the apparent difference in survival between the HBV and NBNC groups.
- A limitation of our study is that the etiologies of NBNC may have differed; they had either non-alcoholic fatty liver disease or alcoholic liver disease. Therefore, we were unable to explain the difference in survival rates between the HBV and NBNC groups. In addition, we did not have information on the cause of death; therefore, we could not distinguish liver-related death due to cancer from other causes. This would have helped to determine whether the high mortality rate in NBNC patients with early-stage cancer was attributable to DM. In addition, we used it as a prognostic indicator. Further studies should consider recurrence-free survival, progression-free survival, tumor response, and quality of life.28 Finally, there were more patients in the HBV group than in the other groups. We initially considered using propensity score matching, but we would have lost a great deal of data if we had used method. Therefore, we decided to preserve the entire dataset.
- In conclusion, the etiology of HCC affected clinical characteristics and prognosis to some extent. NBNC-HCC patients showed shorter OS than viral-related HCC patients. Additionally, the presence of DM is an additional important prognostic factor in patients with early-stage HCC.
DISCUSSION
-
Conflicts of Interest
Hye Won Lee currently serves on the editorial board of J Liver Cancer. She was not involved in the review process of this article. Otherwise, the authors have no conflicts of interest to disclose.
-
Ethics Statement
This study adhered to the ethical guidelines of the Declaration of Helsinki (1975). This study was approved by the Severance Hospital Institutional Review Board (IRB No. 4-2020-1081). The need for informed consent was waived due to the retrospective nature of this study.
-
Funding Statement
This study was supported by the Korean Liver Cancer Association Research Award (2022).
-
Data Availability
The datasets generated or analyzed during this study are available from the corresponding author on reasonable request.
-
Author Contribution
Conceptualization: DYK
Data acquisition: HWL, JSL, BKK, SUK, JYP, SHA, DYK
Data analysis and interpretation: WJJ, HWL, DYK
Writing – original draft: WJJ, HWL, DYK
Writing – review & editing: WJJ, HWL, DYK
Statistical analysis: WJJ, HYL, DYK
Study supervision: DYK
Approval of final manuscript: all authors
Article information
Supplementary Material
Values are expressed as n (%) or median (interquartile range).
AFP, alpha-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer; CCRT, concurrent chemoradiotherapy; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; INR, international normalized ratio; NBNC, non-B non-C; PIVKA-II, prothrombin induced by the absence of vitamin K or antagonist-II; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; TARE, transarterial radioembolization.
HBV, hepatitis B virus; HCV, hepatitis C virus; NBNC, non-B non-C; INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, alpha-fetoprotein; PIVKA-II, prothrombin induced by the absence of vitamin K or antagonist-II; HR, hazard ratio; 95% CI, 95% confidence interval; Ref, reference.
- 1. de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol 2012;56 Suppl 1:S75−S87.PubMed
- 2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893−2917.ArticlePubMed
- 3. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245−1255.ArticlePubMed
- 4. Cantarini MC, Trevisani F, Morselli-Labate AM, Rapaccini G, Farinati F, Del Poggio P, et al. Effect of the etiology of viral cirrhosis on the survival of patients with hepatocellular carcinoma. Am J Gastroenterol 2006;101:91−98.ArticlePubMed
- 5. Kao WY, Su CW, Chau GY, Lui WY, Wu CW, Wu JC. A comparison of prognosis between patients with hepatitis B and C virus-related hepatocellular carcinoma undergoing resection surgery. World J Surg 2011;35:858−867.ArticlePubMedPDF
- 6. Cheon JH, Park JW, Park KW, Kim YI, Kim SH, Lee WJ, et al. The clinical report of 1,078 cases of hepatocellular carcinomas: National Cancer Center experience. Korean J Hepatol 2004;10:288−297.PubMed
- 7. Yoon SK, Chun HG. Status of hepatocellular carcinoma in South Korea. Chin Clin Oncol 2013;2:39. PubMed
- 8. But DY, Lai CL, Yuen MF. Natural history of hepatitis-related hepatocellular carcinoma. World J Gastroenterol 2008;14:1652−1656.ArticlePubMedPMC
- 9. Huang YH, Wu JC, Chen CH, Chang TT, Lee PC, Chau GY, et al. Comparison of recurrence after hepatic resection in patients with hepatitis B vs. hepatitis C-related small hepatocellular carcinoma in hepatitis B virus endemic area. Liver Int 2005;25:236−241.ArticlePubMed
- 10. Gelatti U, Donato F, Tagger A, Fantoni C, Portolani N, Ribero ML, et al. Etiology of hepatocellular carcinoma influences clinical and pathologic features but not patient survival. Am J Gastroenterol 2003;98:907−914.ArticlePubMed
- 11. Llovet JM, Pinyol R, Kelley RK, El-Khoueiry A, Reeves HL, Wang XW, et al. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat Cancer 2022;3:386−401.ArticlePubMedPMCPDF
- 12. Brar G, Greten TF, Graubard BI, McNeel TS, Petrick JL, McGlynn KA, et al. Hepatocellular carcinoma survival by etiology: a SEERmedicare database analysis. Hepatol Commun 2020;4:1541−1551.ArticlePubMedPMCPDF
- 13. Sinn DH, Gwak GY, Cho J, Paik SW, Yoo BC. Comparison of clinical manifestations and outcomes between hepatitis B virus- and hepatitis C virus-related hepatocellular carcinoma: analysis of a nationwide cohort. PLoS One 2014;9:e112184.ArticlePubMedPMC
- 14. Utsunomiya T, Shimada M, Kudo M, Ichida T, Matsui O, Izumi N, et al. A comparison of the surgical outcomes among patients with HBV-positive, HCV-positive, and non-B non-C hepatocellular carcinoma: a nationwide study of 11,950 patients. Ann Surg 2015;261:513−520.PubMed
- 15. Huo TI, Huang YH, Hsia CY, Su CW, Lin HC, Hsu CY, et al. Characteristics and outcome of patients with dual hepatitis B and C-associated hepatocellular carcinoma: are they different from patients with single virus infection? Liver Int 2009;29:767−773.ArticlePubMed
- 16. Kondo K, Chijiiwa K, Funagayama M, Kai M, Otani K, Ohuchida J. Differences in long-term outcome and prognostic factors according to viral status in patients with hepatocellular carcinoma treated by surgery. J Gastrointest Surg 2008;12:468−476.ArticlePubMedPDF
- 17. Chen CH, Huang GT, Yang PM, Chen PJ, Lai MY, Chen DS, et al. Hepatitis B- and C-related hepatocellular carcinomas yield different clinical features and prognosis. Eur J Cancer 2006;42:2524−2529.ArticlePubMed
- 18. Cescon M, Cucchetti A, Grazi GL, Ferrero A, Viganò L, Ercolani G, et al. Role of hepatitis B virus infection in the prognosis after hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a Western dual-center experience. Arch Surg 2009;144:906−913.ArticlePubMed
- 19. Xiao L, Zhang RL, Zhang H, Tulahong A, Zhang YF, Wen H, et al. Comparison of the clinical characteristics and survival between Uyghur patients with hepatitis virus-related and non-B, non-C hepatocellular carcinoma in Xinjiang, China. Chin J Cancer Res 2015;27:279−287.PubMedPMC
- 20. Chen PH, Kao WY, Chiou YY, Hung HH, Su CW, Chou YH, et al. Comparison of prognosis by viral etiology in patients with hepatocellular carcinoma after radiofrequency ablation. Ann Hepatol 2013;12:263−273.ArticlePubMed
- 21. Nishikawa H, Arimoto A, Wakasa T, Kita R, Kimura T, Osaki Y. Comparison of clinical characteristics and survival after surgery in patients with non-B and non-C hepatocellular carcinoma and hepatitis virus-related hepatocellular carcinoma. J Cancer 2013;4:502−513.ArticlePubMedPMC
- 22. Takenaka K, Yamamoto K, Taketomi A, Itasaka H, Adachi E, Shirabe K, et al. A comparison of the surgical results in patients with hepatitis B versus hepatitis C-related hepatocellular carcinoma. Hepatology 1995;22:20−24.ArticlePubMed
- 23. Shiratori Y, Shiina S, Imamura M, Kato N, Kanai F, Okudaira T, et al. Characteristic difference of hepatocellular carcinoma between hepatitis B- and C- viral infection in Japan. Hepatology 1995;22:1027−1033.ArticlePubMed
- 24. Lee SB, Kim KM, An J, Lee D, Shim JH, Lim YS, et al. Clinical characteristics and potential aetiologies of non-B non-C hepatocellular carcinoma in hepatitis B virus endemic area. Liver Int 2016;36:1351−1361.ArticlePubMedPDF
- 25. Su YW, Liu PH, Hsu CY, Lee YH, Hsia CY, Ho SY, et al. Prognostic impact of diabetes mellitus on hepatocellular carcinoma: special emphasis from the BCLC perspective. PLoS One 2017;12:e0174333.ArticlePubMedPMC
- 26. Wang YY, Huang S, Zhong JH, Ke Y, Guo Z, Liu JQ, et al. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma after curative hepatectomy. PLoS One 2014;9:e113858.ArticlePubMedPMC
- 27. Huo TI, Wu JC, Lui WY, Huang YH, Lee PC, Chiang JH, et al. Differential mechanism and prognostic impact of diabetes mellitus on patients with hepatocellular carcinoma undergoing surgical and nonsurgical treatment. Am J Gastroenterol 2004;99:1479−1487.ArticlePubMed
- 28. Sternby Eilard M, Hagström H, Mortensen KE, Wilsgaard T, Vagnildhaug OM, Dajani O, et al. Quality of life as a prognostic factor for survival in hepatocellular carcinoma. Liver Int 2018;38:885−894.ArticlePubMedPDF
References
Figure & Data
References
Citations

- The Epidemiology of Hepatitis B Virus Infection in Korea: 15-Year Analysis
Log Young Kim, Jeong-Ju Yoo, Young Chang, Hoongil Jo, Young Youn Cho, Sangheun Lee, Dong Hyeon Lee, Jae Young Jang
Journal of Korean Medical Science.2024;[Epub] CrossRef - Comparison of Surgical Resection and Radiofrequency Ablation in Elderly Patients with Hepatocellular Carcinoma
Jun Il Kim, Jayoun Lee, Gi Hong Choi, Min Woo Lee, Dong Ah Park, Jeong-Ju Yoo
Digestive Diseases and Sciences.2024; 69(3): 1055. CrossRef - Focal Segmental Glomerulosclerosis Followed by Acute Hepatitis A Infection: Case Report
Min-Woo An, Jeong-Ju Yoo, Jin Kuk Kim, Ahrim Moon, Sang Gyune Kim, Young Seok Kim
Medicina.2023; 59(5): 819. CrossRef - Validation of MELD 3.0 scoring system in East Asian patients with cirrhosis awaiting liver transplantation
Jeong-Ju Yoo, Jong-In Chang, Ji Eun Moon, Dong Hyun Sinn, Sang Gyune Kim, Young Seok Kim
Liver Transplantation.2023; 29(10): 1029. CrossRef - A nationwide study on the current treatment status and natural prognosis of hepatocellular carcinoma in elderly
Jeong-Ju Yoo, Jayoun Lee, Gi Hong Choi, Min Woo Lee, Dong Ah Park
Scientific Reports.2023;[Epub] CrossRef - Statin use and the risk of hepatocellular carcinoma among patients with chronic hepatitis B: an emulated target trial using longitudinal nationwide population cohort data
Dong Hyun Sinn, Danbee Kang, Yewan Park, Hyunsoo Kim, Yun Soo Hong, Juhee Cho, Geum-Youn Gwak
BMC Gastroenterology.2023;[Epub] CrossRef - Addition of Kidney Dysfunction Type to MELD-Na for the Prediction of Survival in Cirrhotic Patients Awaiting Liver Transplantation in Comparison with MELD 3.0 with Albumin
Kyeong-Min Yeom, Jong-In Chang, Jeong-Ju Yoo, Ji Eun Moon, Dong Hyun Sinn, Young Seok Kim, Sang Gyune Kim
Diagnostics.2023; 14(1): 39. CrossRef
 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation
- Download Citation
- Close
- Related articles
-
- The efficacy of treatment for hepatocellular carcinoma in elderly patients
- Stereotactic body radiation therapy for elderly patients with small hepatocellular carcinoma: a retrospective observational study
- A clinical and pathological update on hepatocellular carcinoma
- Is multidisciplinary treatment effective for hepatocellular carcinoma with portal vein tumor thrombus?
- Liver transplantation for hepatocellular carcinoma with portal vein tumor thrombosis

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION


 Follow JLC on Twitter
Follow JLC on Twitter