Articles
- Page Path
- HOME > J Liver Cancer > Volume 22(2); 2022 > Article
-
Original Article
Stereotactic body radiation therapy for elderly patients with small hepatocellular carcinoma: a retrospective observational study -
Jeong Yun Jang1
 , Jinhong Jung1
, Jinhong Jung1 , Danbi Lee2
, Danbi Lee2 , Ju Hyun Shim2
, Ju Hyun Shim2 , Kang Mo Kim2
, Kang Mo Kim2 , Young-Suk Lim2
, Young-Suk Lim2 , Han Chu Lee2
, Han Chu Lee2 , Jin-hong Park1
, Jin-hong Park1 , Sang Min Yoon1
, Sang Min Yoon1
-
Journal of Liver Cancer 2022;22(2):136-145.
DOI: https://doi.org/10.17998/jlc.2022.08.18
Published online: September 16, 2022
1Department of Radiation Oncology, Asan Liver Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
2Department of Gastroenterology, Asan Liver Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
-
Corresponding author: Sang Min Yoon Department of Radiation Oncology, Asan Liver Center, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea
Tel. +82-2-3010-5615, Fax. +82-2-3010-6950 E-mail: drsmyoon@amc.seoul.kr
Copyright © 2022 The Korean Liver Cancer Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 3,486 Views
- 79 Downloads
- 6 Citations
Abstract
-
Background/Aim
- We aimed to investigate the efficacy and safety of stereotactic body radiation therapy (SBRT) in elderly patients with small hepatocellular carcinomas (HCC).
-
Methods
- Eighty-three patients (89 lesions) with HCC who underwent SBRT between January 2012 and December 2018 were reviewed in this retrospective observational study. The key inclusion criteria were as follows: 1) age ≥75 years, 2) contraindications for hepatic resection or percutaneous ablative therapies, 3) no macroscopic vascular invasion, and 4) no extrahepatic metastasis.
-
Results
- The patients were 75-90 years of age, and 49 (59.0%) of them were male. Most patients (94.0%) had an Eastern Cooperative Oncology Group performance status of 0 or 1. Seventy-four patients (89.2%) had Child-Pugh class A hepatic function before SBRT. The median tumor size was 1.6 cm (range, 0.7-3.5). The overall median follow-up period was 34.8 months (range, 7.3-99.3). The 5-year local tumor control rate was 90.1%. The 3-year and 5-year overall survival rate was 57.1% and 40.7%, respectively. Acute toxicity grade ≥3 was observed in three patients (3.6%) with elevated serum hepatic enzymes; however, no patient experienced a worsening of the Child-Pugh score to ≥2 after SBRT. None of the patients developed late toxicity (grade ≥3).
-
Conclusions
- SBRT is a safe treatment option with a high local control rate in elderly patients with small HCC who are not eligible for other curative treatments.
- Primary liver cancer constitutes approximately 75-85% of cases of hepatocellular carcinoma (HCC) and is the sixth most commonly diagnosed cancer and the third leading cause of cancer-related death worldwide in 2020 with approximately 906,000 new cases and 830,000 deaths.1 In the Korean Cancer Statistical Report released in 2022, liver cancer was identified as one of the most commonly diagnosed malignancies in men and women aged 80-84 years and over 85 years, respectively.2 Additionally, according to recent studies from the United States, the United Kingdom, and Japan, the age-specific incidence of HCC significantly increases with age in the population over 75 years of age; data from Korea demonstrated a similar increase in the incidence rate with age.3
- It is well known that one of the major risk factors for HCC is chronic infection with hepatitis B virus (HBV) and hepatitis C virus (HCV). However, the prevalence of HBV has decreased globally due to vaccination, and the increasing prevalence of overweight and diabetes have altered the risk factors for HCC.4 Considering that elderly people tend to have more non-infectious metabolic risk factors, the number of these patients with HCC is expected to increase with global aging.
- Although many treatments have been suggested, several global guidelines have not yet provided specific treatment options for elderly patients with HCC. Active discussion on this topic is ongoing and controversial. Recently, several studies have demonstrated that stereotactic body radiation therapy (SBRT) can deliver a high dose of radiation to the tumor within a short period as an alternative treatment for early-stage HCC.5 However, few studies have reported the efficacy and safety of SBRT in geriatric patients.6,7 Therefore, in the present study, we investigated our clinical data to evaluate the feasibility of SBRT in patients of advanced age.
INTRODUCTION
- 1. Patients
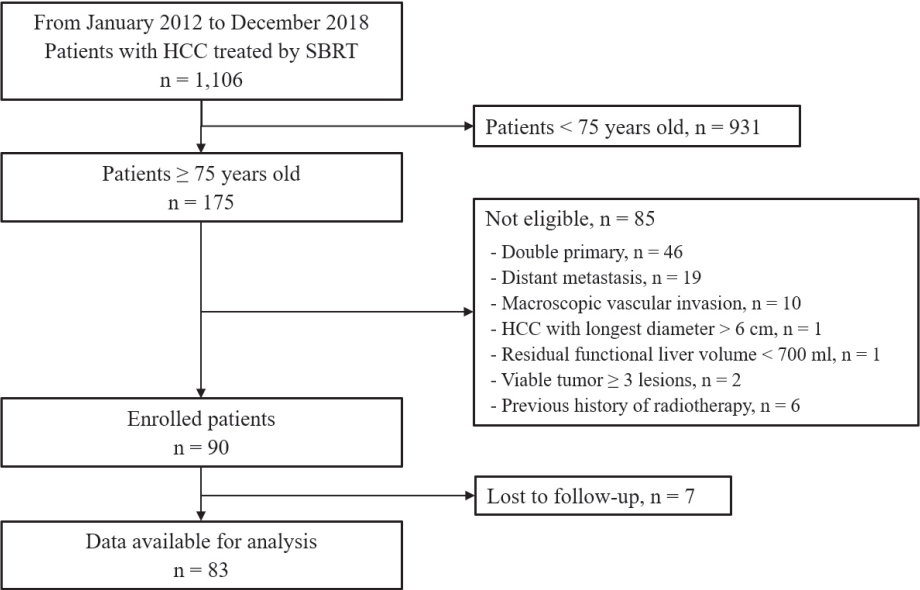
- In this retrospective observational study, we reviewed the medical records of 1,106 patients who underwent SBRT for HCC at Asan Medical Center between January 2012 and December 2018. In the present study, elderly age was defined as age of 75 years or older, and 175 patients met this criterion. Of them, 90 patients satisfied the following inclusion criteria: 1) contraindications for curative treatments, such as hepatic resection, transplantation, or radiofrequency ablation (RFA) due to medical and technical limitations or preferences of the patients; 2) HCC with the largest diameter ≤6 cm; 3) two or fewer viable tumors; 4) no evidence of distant metastasis; 5) no macroscopic vascular invasion; 6) no other malignancies at the time of diagnosis of HCC; 7) adequate residual liver volume (>700 mL); 8) sufficient distance between HCC and surrounding normal organs; and 9) no previous history of radiation therapy in the liver. At the final follow-up, 83 patients were analyzed and 89 lesions were treated (Fig. 1). The Strengthening and Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed for this study (Supplementary Table 1).
- The diagnosis of HCC was based on histological confirmation and/or typical findings on multi-phase dynamic computed tomography (CT) or magnetic resonance imaging (MRI) using a hepatocyte-specific contrast agent according to the practice guidelines of the Korean Liver Cancer Association - National Cancer Center Korea.8 This study was approved by the Institutional Review Board (IRB) of Asan Medical Center (IRB No. #2021-0280), and the requirement for written informed consent was waived owing to the retrospective design.
- 2. Radiation therapy
- Four-dimensional (4D) CT (GE LightSpeed RT 16; GE Healthcare, Waukesha, WI, USA) simulation with freebreathing was performed 3-4 days before initiating SBRT with the patient in a supine position with both arms raised on the wing board. The 4D-CT images were sorted into 10 series using respiratory data (Advantage 4D version 4.2; GE Healthcare).9 The respiratory data were analyzed using a Real-time Position Management gating system (Varian Medical Systems, Palo Alto, CA, USA).9,10
- The gross tumor volume (GTV) was delineated using dynamic contrast CT or MRI in the end-expiratory phase of the 4D-CT images. The internal target volume (ITV) was set by considering GTV movements within the specific amplitude of breathing. For the planning target volume (PTV), a margin of 5 mm was added in all directions from the ITV. SBRT planning was performed using a three-dimensional treatment planning system (Eclipse; Varian Medical Systems). The two arcs of the volumetric-modulated arc therapy technique using a 10-MV flattening filter-free beam with a linear accelerator (TrueBeam STx; Varian Medical Systems).9 The median total dose was 45 Gy (range, 36-60), and the fraction size was either 12 or 15 Gy. The total dose was determined according to prescription guidelines described previously.9 SBRT was delivered once daily for three or four consecutive days using kV cone beam CT guidance and setup correction based on the liver dome, compact iodized oil, surgical clips, or gold fiducial markers.
- 3. Evaluation and statistical analysis
- Baseline evaluation before SBRT included history taking, physical examination, blood tests, including complete blood count, liver function tests, tumor markers, and multi-phase dynamic CT and/or MRI. During the treatment period, patients were examined by a radiation oncologist for any adverse events during SBRT. Follow-up examinations were performed regularly at 2-3-month intervals.
- Response evaluation was performed according to both the Response Evaluation Criteria in Solid Tumor (RECIST) version 1.1, and modified RECIST (mRECIST) criteria based on imaging 3 months after the treatment, and all images were reviewed until the best response according to the mRECIST criteria was noted.11,12 Radiological response was defined as the proportion of lesions with complete and partial responses. Treatment-related toxicity was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE; version 5.0). Radiation-induced liver disease was defined as an increase in serum liver enzyme levels according to CTCAE or an increase in Child-Pugh score by ≥2 points with no progressive disease within 3 months after SBRT.
- Local control was defined as no HCC regrowth or recurrence in the PTV despite an initial response confirmed by CT or MRI. Overall survival (OS) and intrahepatic recurrence-free survival (IHRFS) rates were defined as the duration between the first day of SBRT and the date of death, last followup examination, or the date of recurrence within the liver outside the SBRT field. The probability of cumulative survival was calculated using the Kaplan-Meier method. Univariate analysis was also performed using the log-rank test to determine the risk factors for IHRFS and OS with P-values set to 0.05. All statistical analyses were performed using SPSS version 21 (IBM SPSS Statistics, Armonk, NY, USA).
METHODS
- 1. Patient characteristics
- A total of 83 patients were analyzed over a median followup period of 34.8 months (range, 7.3-99.3), and their characteristics are summarized in Table 1. The median age was 77 years (range, 75-90), and most patients had a good Eastern Cooperative Oncology Group performance status (ECOG PS) of 0-1. However, one patient had a hip fracture due to accidental fall 5 months before SBRT, and orthopedic surgery was impossible due to old age, which resulted in ECOG PS of 3. Additionally, 57 patients (68.7%) had HCC secondary to HBV or HCV, and non-viral etiologies accounted for 31.3% of cases. Nine patients (10.8%) had Child-Pugh class B hepatic function (Child-Pugh scores of 7, 8, and 9 in two, five, and two patients, respectively). The median tumor size was 1.6 cm (range, 0.7-3.5), and most lesions were smaller than 3 cm. The diverse population included treatment-naïve patients who were never treated for HCC as well as those who had received up to 18 different locoregional treatments for HCC.
- 2. Local control and response rate
- Local failure was noted in only 5/89 lesions. The local control rates at 3- and 5-year were 97.7% and 90.1%, respectively (Fig. 2A). An example of a patient with local failure is presented in Supplementary Fig. 1.
- Three months after SBRT, radiologic responses were observed in 60 (67.4%) and 69 (77.6%) lesions according to the RECIST and modified RECIST criteria, respectively (Table 2). Evaluation was conducted until the best response, and 96.6% of the lesions revealed a radiologic response at a median of 4.2 months (range, 0.9-12.4) after completion of SBRT (Table 2). No dose-response relationship was observed in this analysis.
- 3. Overall and recurrence-free survival rates
- At the last follow-up, 34 patients survived. The median OS was 45.4 months (95% confidence interval [CI], 35.3-55.5), with 3- and 5-year OS rates of 57.1% and 40.7%, respectively (Fig. 2B). Intrahepatic recurrence was observed in 52 patients during the follow-up. The median IHRFS was 15.8 months (95% CI, 10.2-21.4), and the 3- and 5-year IHRFS rates were 29.5% and 10.4%, respectively (Fig. 2C). Univariate analysis revealed that Child-Pugh class was significantly associated with OS (3-year survival rate, Child-Pugh class A: 61.4%, Child-Pugh class B: 22.2%; P=0.001). The serum alpha-fetoprotein (AFP) level before SBRT was related to the IHRFS (3-year survival rate, AFP <20 ng/mL: 49.9%, AFP ≥20 ng/mL: 21.2%; P=0.029) (Table 3).
- 4. Treatment-related toxicities
- SBRT-related toxicities are summarized in Table 4. Acute toxicities were observed in 42 patients (50.6%); however, most were evaluated as grade 1 or 2 abnormal laboratory findings or constitutional symptoms. Therefore, there were no intolerable adverse events to SBRT in any of the patients. Although grade 3 hepatic enzyme elevation was noted in three patients (3.6%), these abnormalities improved without any intervention. Late toxicities were noted in 31 patients (37.3%) (30 patients with grade 1 and one patient with grade 2 toxicity). No late toxicity beyond grade 3 was observed. One patient complained of dry cough due to radiation pneumonitis (grade 2); however, it improved with oral medications. Rib fractures were also noted in six patients with grade 1 toxicity; they were simple fractures identified on CT that healed without any special treatment.
RESULTS
- In this retrospective study of SBRT in elderly patients (≥ 75 years of age) with HCC, we observed similar local control and OS rates (90.1% and 40.7% at 5 years, respectively) and minimal treatment-related toxicity in comparison with the clinical outcomes of SBRT in all age groups.13
- Elderly patients with HCC have distinct features and clinical implications than younger patients, and preclinical data suggest that different genetic mechanisms are involved in the development of HCC according to age.14 First, the proportion of female patients is high.15,16 In this study, 41% of the patients were female, which is much higher than the rate reported in other studies that enrolled patients of all ages.13 Second, regarding oncological characteristics, the rate of encapsulated, unifocal, and well-differentiated tumors is more common in the elderly, which may be a favorable prognostic factor.16-18 Third, in terms of etiology, there are more non-B and non-C patients, especially those with non-alcoholic steatohepatitis.16 Up to 46% of these patients have a non-cirrhotic background, and they have etiologies different from HCC related to chronic hepatitis, which is characterized by a unique tumor microenvironment and response to treatments.19 Fourth, elderly patients have a smaller liver volume and unsatisfactory liver metabolism and cell regeneration capacity than younger patients.20,21 Finally, in terms of radiation therapy procedures, it is usually difficult to control respiration during SBRT in elderly patients. Therefore, 4D-CT simulation with free-breathing and respiratory-gated SBRT by setting a specific amplitude would be an appropriate approach in the elderly. Therefore, these unique characteristics are sometimes seen as good for patient prognosis but sometimes it is difficult to select the optimal treatment method.
- Few studies have investigated SBRT for HCC in elderly patients. Teraoka et al.7 defined the inclusion criteria for the elderly group as age >75 years, ECOG PS of 0-1, Child-Pugh score ≤7, HCC with <3 nodules of up to 3 cm, and no vascular invasion. The 3-year local control, progression-free survival (PFS), and OS rates were 98.1%, 29.2%, and 57.1%, respectively, which are similar to our results. In this Japanese study, an excellent local control rate was also observed irrespective of age; therefore, the clinical outcomes in the elderly patients at our institution are comparable to those in younger patients. Another SBRT study by Loi et al.6 enrolled patients aged >80 years and reported 2-year local control, PFS, and OS rates of 93%, 31%, and 43%, respectively. This study included more patients with Child-Pugh class B than our study, and almost half of the patients (51%) had tumor size ≥3 cm.
- Studies on hepatic resection or RFA in elderly patients have been pursued more actively than radiation therapy, and conflicting results have been published. In surgical studies, most of the cut-off age values were 70 or 75 years, and the 3-year disease-free survival (DFS) and OS rates varied from 36% to 58% and 55% to 73%, respectively.17,22-29 Although most studies reported no difference in the survival rates between older and younger patients,22,23,27,29 one study reported impaired OS rates following hepatic resection in elderly patients.28 RFA was also performed in patients aged >70 or 75 years, and the results demonstrated DFS and OS rates of 21-49% and 51-82%, respectively, with inconsistent conclusions between several studies regarding the effects of age.24,30,31 Fujiwara et al.30 found that postoperative management of infectious and cardiovascular diseases affected the survival rate of elderly patients. Since both surgery and RFA include risks of bleeding or infection, the increased risk due to invasiveness itself is likely to be pronounced in the elderly, which emphasizes the need for careful selection of patients.
- Due to the characteristics of HCC, intrahepatic recurrence frequently occurs at other sites, even if one lesion is well-controlled.32 In this study, 52 cases (62.7%) of intrahepatic recurrence were noted during the follow-up period. On univariate analysis of IHRFS, the number of previous treatments was found to be marginally significant. Conversely, it was possible to confirm that patients who received several other treatments in the past had a higher risk of intrahepatic recurrence than those who did not. The Child-Pugh class was identified as a factor that favored OS rates, and liver function has already been proven to be an important prognostic factor related to the survival of patients.33-35
- Acute toxicities after SBRT in elderly patients with HCC were mostly mild (grade 1-2), which is similar to the results of a previous study on SBRT for small (≤5 cm) HCC in all age groups.9 Although three patients (3.6%) experienced grade 3 serum hepatic enzyme elevation after SBRT, they recovered well without any intervention. Regarding late toxicities, the incidence of radiation pneumonitis (28.9%) was relatively higher than that (12%) in our previous study;9 however, this finding might be related to the location of the HCC (e.g., HCC located in the liver dome). Furthermore, most cases of radiation pneumonitis did not cause any symptoms (only one patient experienced dry cough); this might not be considered a clinically significant adverse event in elderly patients with HCC after SBRT. The incidence of rib fracture was slightly lower than that in our previous study (7.2% vs. 10%, respectively),9 but it is difficult to compare directly because the incidence of rib fracture is also strongly related to the location of HCC. Compared to previous studies that included elderly patients,6,7 the incidence and severity of toxicities appear to be similar. Therefore, SBRT is a safe treatment even in elderly patients with HCC.
- The present study had some limitations. First, there was no control group of younger patients. When designing this study, we aimed to include a control group by performing propensity score matching. However, age includes several confounding variables and all factors cannot be sufficiently corrected. Therefore, we tried to overcome this issue by indirectly comparing our results with those of other SBRT studies in all age groups at our institution. Second, we enrolled patients in this study based on their chronological age, which does not necessarily reflect the biological and functional status of the patients. We wonder if novel results will be obtained if a study is designed with consideration of each patient’s geriatric condition. There are various measuring tools related to the functional evaluation of the elderly, including the Geriatric 8 (G8) and Vulnerable Elders-13 Survey.36,37 Loi et al.6 published the only study that examined the relationship between the clinical outcomes of SBRT and G8 and the Charlson comorbidity index (CCI) in geriatric patients. Those with impairments in the G8 and CCI domains were reported to have a lower survival rate and a rapid onset of toxicity. Therefore, it is important to select a personalized treatment option along with the analysis of detailed factors that are known to affect the prognosis in elderly patients, such as body mass index, neuropsychological problems, degree of weight loss, or type of mobility. For this analysis to be possible, thorough records and data regarding various items from before treatment must be collected, and it will be meaningful to conduct such a study in the future.
- In conclusion, SBRT is a good treatment option with high response and local control rates and a lower incidence of severe toxicity in elderly patients with HCC when other curative treatments cannot be safely administered.
DISCUSSION
-
Conflicts of Interest
The authors have no conflicts to disclose.
-
Ethics Statement
This study was approved by the Institutional Review Board (IRB) of Asan Medical Center (IRB No. #2021-0280), and the requirement for written informed consent was waived owing to the retrospective design. This study was conducted in accordance with the 1964 Declaration of Helsinki.
-
Funding Statement
None.
-
Author Contribution
Conceptualization: JYJ, JJ, SMY
Acquisition of data: JYJ, SMY
Analysis and interpretation: JYJ, JJ, JHS, JP, SMY
Drafting of the manuscript: JYJ, SMY
Supervision: DL, KMK, YL, HCH, SMY
Approval of final manuscript: all authors
Article information
Supplementary Material

| Variable | Value |
|---|---|
| Age (years) | 77 (75-90) |
| Sex | |
| Male | 49 (59.0) |
| Female | 34 (41.0) |
| ECOG performance status | |
| 0 | 72 (86.8) |
| 1 | 6 (7.2) |
| 2-3 | 5 (6.0) |
| Etiology | |
| Hepatitis B virus infection | 34 (41.0) |
| Hepatitis C virus infection | 23 (27.7) |
| Others | 26 (31.3) |
| Child-Pugh class | |
| A | 74 (89.2) |
| Child-Pugh score, 5 | 41 (49.4) |
| Child-Pugh score, 6 | 33 (39.8) |
| B | 9 (10.8) |
| Child-Pugh score, 7 | 2 (2.4) |
| Child-Pugh score, 8 | 5 (6.0) |
| Child-Pugh score, 9 | 2 (2.4) |
| Tumor size (cm)* | 1.6 (0.7-3.5) |
| 0.7-1.0 | 14 (15.7) |
| 1.1-2.0 | 53 (59.6) |
| 2.1-3.0 | 19 (21.3) |
| 3.1-4.0 | 3 (3.4) |
| Alpha-fetoprotein (ng/mL) | 10.25 (0.97-1191.3) |
| PIVKA-II (mAU/mL)† | 24 (10-56007) |
| Number of prior treatments before SBRT | 3 (0-18) |
| Summary of prior treatments‡ | |
| None | 3 (3.6) |
| TACE | 28 (33.8) |
| TACE, RFA (± PEI) | 24 (28.9) |
| Resection, TACE | 13 (15.7) |
| Resection, TACE, RFA | 7 (8.4) |
| Resection, RFA | 4 (4.8) |
| RFA | 4 (4.8) |
Values are presented as number (%) or median (range).
ECOG, Eastern Cooperative Oncology Group; PIVKA, protein induced by vitamin K absence or antagonist; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; PEI, percutaneous ethanol injection.
* Eighty-nine tumors were evaluated, and the size of all tumors was measured according to the modified RECIST;
† PIVKA-II examination was not performed in 5 patients;
‡ Prior treatments before SBRT includes hepatic resection, radiofrequency ablation, percutaneous ethanol injection, and transarterial chemoembolization/chemoinfusion.
|
RECIST |
mRECIST |
||
|---|---|---|---|
| At 3 months | At 3 months | Best response | |
| Overall response rates* | 60 (67.4) | 69 (77.6) | 86 (96.6) |
| Complete response | 30 (33.7) | 45 (50.6) | 82 (92.1) |
| Partial response | 30 (33.7) | 24 (27.0) | 4 (4.5) |
| Stable disease | 28 (31.5) | 19 (21.3) | 2 (2.3) |
| Progressive disease | 1 (1.1) | 1 (1.1) | 1 (1.1) |
|
Intrahepatic recurrence-free survival |
Overall survival |
|||
|---|---|---|---|---|
| 3-year rate (%) (Survivors/No. of patients)* | P-value | 3-year rate (%) (Survivors/No. of patients)* | P-value | |
| Sex | ||||
| Male | 43.7 (23/49) | 0.947 | 63.3 (31/49) | 0.639 |
| Female | 35.3 (15/34) | 48.1 (17/34) | ||
| Age | ||||
| <80 years | 42.3 (30/63) | 0.482 | 58.7 (37/63) | 0.929 |
| ≥80 years | 37.1 (8/20) | 50.6 (11/20) | ||
| ECOG PS | ||||
| 0-1 | 41.3 (36/78) | 0.531 | 56.9 (45/78) | 0.613 |
| 2-3 | 26.7 (2/5) | 60.0 (3/5) | ||
| Etiology | ||||
| Viral | 39.9 (25/57) | 0.228 | 62.6 (36/57) | 0.394 |
| Non-viral | 38.4 (13/26) | 44.7 (12/26) | ||
| Child-Pugh class | ||||
| A | 43.2 (35/74) | 0.504 | 61.4 (46/74) | 0.001 |
| B | 17.8 (3/9) | 22.2 (2/9) | ||
| Tumor size | ||||
| <1.6 cm | 40.2 (15/37) | 0.233 | 55.4 (21/37) | 0.916 |
| ≥1.6 cm | 41.0 (23/46) | 58.5 (27/46) | ||
| Alpha-fetoprotein | ||||
| <20 ng/mL | 49.9 (31/56) | 0.029 | 61.6 (35/56) | 0.317 |
| ≥20 ng/mL | 21.2 (7/27) | 47.9 (13/27) | ||
| PIVKA-II | ||||
| <40 mAU/mL | 41.5 (27/58) | 0.643 | 57.5 (34/58) | 0.387 |
| ≥40 mAU/mL | 39.1 (11/25) | 56.0 (14/25) | ||
| No. of prior treatment | ||||
| <3 times | 59.1 (23/37) | 0.051 | 64.2 (24/37) | 0.200 |
| ≥3 times | 25.3 (15/46) | 51.1 (24/46) | ||
| EQD2 | ||||
| <93.8 Gy | 26.7 (3/6) | 0.840 | 50.0 (3/6) | 0.564 |
| ≥93.8 Gy | 41.3 (35/77) | 57.7 (45/77) | ||
| Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Acute toxicities | ||||
| Fatigue | 9 (10.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Anorexia | 5 (6.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nausea | 5 (6.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dyspepsia | 1 (1.2) | 1 (1.2) | 0 (0.0) | 0 (0.0) |
| Abdominal pain | 2 (2.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AST/ALT elevation | 22 (26.5) | 0 (0.0) | 3 (3.6) | 0 (0.0) |
| Alkaline phosphatase elevation | 9 (10.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Bilirubin elevation | 14 (16.9) | 1 (1.2) | 0 (0.0) | 0 (0.0) |
| Late toxicities | ||||
| Radiation pneumonitis | 23 (27.7) | 1 (1.2) | 0 (0.0) | 0 (0.0) |
| Rib fracture* | 6 (7.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Biliary stricture | 4 (4.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Values are presented as number (%).
AST, aspartate transaminase; ALT, alanine transaminase.
* Grade 1, simple healed fracture; grade 2, dislocation exceeding half of the rib diameter; and grade 3, rib fracture with associated myositis, according to the proposed grading system at Asan Medical Center.
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209−249.ArticlePubMedPDF
- 2. Kang MJ, Won YJ, Lee JJ, Jung KW, Kim HJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2019. Cancer Res Treat 2022;54:330−344.ArticlePubMedPMCPDF
- 3. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557−2576.ArticlePubMed
- 4. Marengo A, Rosso C, Bugianesi E. Liver cancer: connections with obesity, fatty liver, and cirrhosis. Annu Rev Med 2016;67:103−117.ArticlePubMed
- 5. Apisarnthanarax S, Barry A, Cao M, Czito B, DeMatteo R, Drinane M, et al. External beam radiation therapy for primary liver cancers: an ASTRO clinical practice guideline. Pract Radiat Oncol 2022;12:28−51.ArticlePubMed
- 6. Loi M, Comito T, Franzese C, Desideri I, Dominici L, Lo Faro L, et al. Charlson comorbidity index and G8 in older old adult(≥80 years) hepatocellular carcinoma patients treated with stereotactic body radiotherapy. J Geriatr Oncol 2021;12:1100−1103.ArticlePubMed
- 7. Teraoka Y, Kimura T, Aikata H, Daijo K, Osawa M, Honda F, et al. Clinical outcomes of stereotactic body radiotherapy for elderly patients with hepatocellular carcinoma. Hepatol Res 2018;48:193−204.ArticlePubMedPDF
- 8. Korean Liver Cancer Association, National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver 2019;13:227−299.ArticlePubMedPMC
- 9. Yoon SM, Kim SY, Lim YS, Kim KM, Shim JH, Lee D, et al. Stereotactic body radiation therapy for small (≤5 cm) hepatocellular carcinoma not amenable to curative treatment: results of a singlearm, phase II clinical trial. Clin Mol Hepatol 2020;26:506−515.ArticlePubMedPMCPDF
- 10. Park S, Jung J, Cho B, Kim SY, Yun SC, Lim YS, et al. Clinical outcomes of stereotactic body radiation therapy for small hepatocellular carcinoma. J Gastroenterol Hepatol 2020;35:1953−1959.ArticlePubMedPDF
- 11. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228−247.ArticlePubMed
- 12. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52−60.ArticlePubMed
- 13. Yoon SM, Lim YS, Park MJ, Kim SY, Cho B, Shim JH, et al. Stereotactic body radiation therapy as an alternative treatment for small hepatocellular carcinoma. PLoS One 2013;8:e79854.ArticlePubMedPMC
- 14. Colak D, Chishti MA, Al-Bakheet AB, Al-Qahtani A, Shoukri MM, Goyns MH, et al. Integrative and comparative genomics analysis of early hepatocellular carcinoma differentiated from liver regeneration in young and old. Mol Cancer 2010;9:146. ArticlePubMedPMC
- 15. Kim YJ, Jang BK, Kim ES, Chung WJ, Park KS, Cho KB, et al. Hepatocellular carcinoma in the elderly: clinical characteristics, treatment, survival analysis in Korean patients older than 70 years. J Korean Med Sci 2012;27:1147−1154.ArticlePubMedPMCPDF
- 16. Honda T, Miyaaki H, Ichikawa T, Taura N, Miuma S, Shibata H, et al. Clinical characteristics of hepatocellular carcinoma in elderly patients. Oncol Lett 2011;2:851−854.PubMedPMC
- 17. Huang J, Li BK, Chen GH, Li JQ, Zhang YQ, Li GH, et al. Long-term outcomes and prognostic factors of elderly patients with hepatocellular carcinoma undergoing hepatectomy. J Gastrointest Surg 2009;13:1627−1635.ArticlePubMedPDF
- 18. Okuda K, Musha H, Nakajima Y, Kubo Y, Shimokawa Y, Nagasaki Y, et al. Clinicopathologic features of encapsulated hepatocellular carcinoma: a study of 26 cases. Cancer 1977;40:1240−1245.ArticlePubMed
- 19. Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol 2019;16:411−428.ArticlePubMedPDF
- 20. Schmucker DL. Age-related changes in liver structure and function: Implications for disease ? Exp Gerontol 2005;40:650−659.ArticlePubMed
- 21. Wynne HA, Cope LH, Mutch E, Rawlins MD, Woodhouse KW, James OF. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology 1989;9:297−301.ArticlePubMed
- 22. Hirokawa F, Hayashi M, Miyamoto Y, Asakuma M, Shimizu T, Komeda K, et al. Surgical outcomes and clinical characteristics of elderly patients undergoing curative hepatectomy for hepatocellular carcinoma. J Gastrointest Surg 2013;17:1929−1937.ArticlePubMedPDF
- 23. Iida H, Kaibori M, Matsui K, Ishizaki M, Kon M. Assessing the feasibility of clinicopathological features of hepatic resection for hepatocellular carcinoma in patients over 80 years of age. Mol Clin Oncol 2017;6:29−38.ArticlePubMedPMC
- 24. Mirici-Cappa F, Gramenzi A, Santi V, Zambruni A, Di Micoli A, Frigerio M, et al. Treatments for hepatocellular carcinoma in elderly patients are as effective as in younger patients: a 20-year multicentre experience. Gut 2010;59:387−396.ArticlePubMed
- 25. Nishikawa H, Arimoto A, Wakasa T, Kita R, Kimura T, Osaki Y. Surgical resection for hepatocellular carcinoma: clinical outcomes and safety in elderly patients. Eur J Gastroenterol Hepatol 2013;25:912−919.PubMed
- 26. Oishi K, Itamoto T, Kobayashi T, Oshita A, Amano H, Ohdan H, et al. Hepatectomy for hepatocellular carcinoma in elderly patients aged 75 years or more. J Gastrointest Surg 2009;13:695−701.ArticlePubMedPDF
- 27. Poon RT, Fan ST, Lo CM, Liu CL, Ngan H, Ng IO, et al. Hepatocellular carcinoma in the elderly: results of surgical and nonsurgical management. Am J Gastroenterol 1999;94:2460−2466.ArticlePubMed
- 28. Santambrogio R, Barabino M, Scifo G, Costa M, Giovenzana M, Opocher E. Effect of age (over 75 Years) on postoperative complications and survival in patients undergoing hepatic resection for hepatocellular carcinoma. J Gastrointest Surg 2017;21:657−665.ArticlePubMedPDF
- 29. Sato S, Tanaka K, Nojiri K, Kumamoto T, Mori R, Taniguchi K, et al. Hepatic resection for hepatocellular carcinoma in the elderly: selecting hepatectomy procedures based on patient age. Anticancer Res 2015;35:6855−6860.PubMed
- 30. Fujiwara N, Tateishi R, Kondo M, Minami T, Mikami S, Sato M, et al. Cause-specific mortality associated with aging in patients with hepatocellular carcinoma undergoing percutaneous radiofrequency ablation. Eur J Gastroenterol Hepatol 2014;26:1039−1046.ArticlePubMed
- 31. Takahashi H, Mizuta T, Kawazoe S, Eguchi Y, Kawaguchi Y, Otuka T, et al. Efficacy and safety of radiofrequency ablation for elderly hepatocellular carcinoma patients. Hepatol Res 2010;40:997−1005.ArticlePubMed
- 32. Cheng Z, Yang P, Qu S, Zhou J, Yang J, Yang X, et al. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB (Oxford) 2015;17:422−427.ArticlePubMedPMC
- 33. Brown DB, Fundakowski CE, Lisker-Melman M, Crippin JS, Pilgram TK, Chapman W, et al. Comparison of MELD and Child-Pugh scores to predict survival after chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol 2004;15:1209−1218.ArticlePubMed
- 34. Kudo M, Osaki Y, Matsunaga T, Kasugai H, Oka H, Seki T. Hepatocellular carcinoma in Child-Pugh C cirrhosis: prognostic factors and survival benefit of nontransplant treatments. Dig Dis 2013;31:490−498.ArticlePubMedPDF
- 35. Pressiani T, Boni C, Rimassa L, Labianca R, Fagiuoli S, Salvagni S, et al. Sorafenib in patients with Child-Pugh class A and B advanced hepatocellular carcinoma: a prospective feasibility analysis. Ann Oncol 2013;24:406−411.ArticlePubMed
- 36. Min L, Yoon W, Mariano J, Wenger NS, Elliott MN, Kamberg C, et al. The vulnerable elders-13 survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. J Am Geriatr Soc 2009;57:2070−2076.ArticlePubMedPMC
- 37. Takahashi M, Takahashi M, Komine K, Yamada H, Kasahara Y, Chikamatsu S, et al. The G8 screening tool enhances prognostic value to ECOG performance status in elderly cancer patients: a retrospective, single institutional study. PLoS One 2017;12:e0179694.ArticlePubMedPMC
References
Figure & Data
References
Citations

- Radiofrequency Ablation versus Surgical Resection in Elderly Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
Jeong-Ju Yoo, Sujin Koo, Gi Hong Choi, Min Woo Lee, Seungeun Ryoo, Jungeun Park, Dong Ah Park
Current Oncology.2024; 31(1): 324. CrossRef - Efficacy and Safety of Surgical Resection in Elderly Patients with Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
Jin-Soo Lee, Dong Ah Park, Seungeun Ryoo, Jungeun Park, Gi Hong Choi, Jeong-Ju Yoo
Gut and Liver.2024; 18(4): 695. CrossRef - Radiotherapy trend in elderly hepatocellular carcinoma: retrospective analysis of patients diagnosed between 2005 and 2017
Bong Kyung Bae, Jeong Il Yu, Hee Chul Park, Myung Ji Goh, Yong-Han Paik
Radiation Oncology Journal.2023; 41(2): 98. CrossRef - Loco-regional therapies competing with radiofrequency ablation in potential indications for hepatocellular carcinoma: a network meta-analysis
Ha Il Kim, Jihyun An, Seungbong Han, Ju Hyun Shim
Clinical and Molecular Hepatology.2023; 29(4): 1013. CrossRef - Has the growing evidence of radiotherapy for hepatocellular carcinoma increased the use of radiotherapy in elderly patients?
Tae Hyun Kim
Radiation Oncology Journal.2023; 41(3): 141. CrossRef - Chronic Liver Disease in the Older Patient—Evaluation and Management
Daniel Anthony DiLeo, Tolga Gidener, Ayse Aytaman
Current Gastroenterology Reports.2023; 25(12): 390. CrossRef
 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation
- Download Citation
- Close
- Related articles
-
- Current perspectives on radiotherapy in hepatocellular carcinoma management: a comprehensive review
- Additional nodules detected using EOB-MRI in patients with resectable single hepatocellular carcinoma: an implication for active treatment strategy
- Letter regarding “Treatment options for solitary hepatocellular carcinoma ≤5 cm: surgery vs. ablation: a multicenter retrospective study”
- Treatment options for solitary hepatocellular carcinoma ≤5 cm: surgery vs. ablation: a multicenter retrospective study
- Letter regarding “Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab”

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION


 Follow JLC on Twitter
Follow JLC on Twitter