Articles
- Page Path
- HOME > J Liver Cancer > Volume 22(2); 2022 > Article
-
Original Article
Effect of direct-acting antivirals for hepatitis C virus-related hepatocellular carcinoma recurrence and death after curative treatment -
Young-Hwan Ahn1*
 , Heirim Lee2,3*
, Heirim Lee2,3* , Ji Eun Han1
, Ji Eun Han1 , Hyo Jung Cho1
, Hyo Jung Cho1 , Jae Youn Cheong1
, Jae Youn Cheong1 , Bumhee Park2,3
, Bumhee Park2,3 , Soon Sun Kim1
, Soon Sun Kim1
-
Journal of Liver Cancer 2022;22(2):125-135.
DOI: https://doi.org/10.17998/jlc.2022.05.24
Published online: June 28, 2022
1Department of Gastroenterology, Ajou University School of Medicine, Suwon, Korea
2Department of Biomedical Informatics, Ajou University School of Medicine, Suwon, Korea
3Office of Biostatistics, Medical Research Collaborating Center, Ajou Research Institute for Innovative Medicine, Ajou University Medical Center, Suwon, Korea
-
Co-corresponding author: Bumhee Park Department of Biomedical Informatics, Ajou University School of Medicine, 164 World cup-ro, Yeongtong-gu, Suwon 16499, Korea
Tel. +82-31-219-4458, Fax. +82-31-219-4472 E-mail: bhpark@ajou.ac.kr -
Corresponding author: Soon Sun Kim Department of Gastroenterology, Ajou University School of Medicine, 164 World cup-ro, Yeongtong-gu, Suwon 16499, Korea
Tel. +82-31-219-7822, Fax. +82-31-219-7820 E-mail: cocorico99@gmail.com - *These two authors contributed equally to this work.
Copyright © 2022 The Korean Liver Cancer Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 3,531 Views
- 86 Downloads
- 4 Citations
Abstract
-
Background/Aim
- There has been a long-standing debate about the association of directacting antiviral (DAA) therapy and hepatocellular carcinoma (HCC) recurrence. This study aimed to investigate the association between DAA therapy and HCC recurrence after curative therapy.
-
Methods
- We retrospectively enrolled 1,021 patients with HCV-related (hepatitis C virus) HCC who underwent radiofrequency ablation (RFA), liver resection, or both as the first treatment modality from January 2007 to December 2016 and without a history of HCV therapy before HCC treatment from a nationwide database. The effect of HCV treatment on HCC recurrence and all-cause mortality was also investigated.
-
Results
- Among the 1,021 patients, 77 (7.5%) were treated with DAA, 14 (1.4%) were treated with interferon-based therapy, and 930 (91.1%) did not receive HCV therapy. DAA therapy was an independent prognostic factor for lower HCC recurrence rate (hazard ratio [HR], 0.04; 95% confidence interval [CI], 0.006-0.289; P=0.001 for landmarks at 6 months after HCC treatment and HR, 0.05; 95% CI, 0.007-0.354; P=0.003 for landmarks at 1 year). Furthermore, DAA therapy was associated with lower all-cause mortality (HR, 0.049; 95% CI, 0.007-0.349; P=0.003 for landmarks at 6 months and HR, 0.063; 95% CI, 0.009-0.451; P=0.006 for landmarks at 1 year).
-
Conclusions
- DAA therapy after curative HCC treatment can decrease HCC recurrence and all-cause mortality compared to interferon-based therapy or no antiviral therapy. Therefore, clinicians should consider administering DAA therapy after curative HCC treatment in patients with HCV-related HCC.
- Since the introduction of direct-acting antiviral agents (DAAs) in the past decade, there has been remarkable development in the treatment of chronic hepatitis C. In the 2000s, a pegylated interferon (IFN)-based treatment regimen showed a virologic response of 40-70%. In the DAA era, the reported rate of sustained virologic response at 12 weeks is 90-98%, even in patients with advanced liver cirrhosis.1-4 However, 71 million people are estimated to remain infected with hepatitis C virus (HCV). Chronic hepatitis C is considered a leading cause of liver cirrhosis and hepatocellular carcinoma (HCC), especially in Western countries.5,6 HCC is ranked the third most common cause of cancer-related deaths, and its incidence is expected to increase until 2030.7
- Although there is no doubt that DAA therapy should be actively conducted to reduce the morbidity and mortality rate from HCC, whether DAA therapy should be applied to patients with HCV-related HCC is debated. Since the report of an unexpectedly high rate of early HCC recurrence in patients with HCV-related HCC after DAA therapy, several reports have made clinicians hesitant to recommend expensive DAA therapy to patients with HCC after curative treatment.8 A study on Italian HCC cohorts reported that after curative treatment, patients had a high rate of HCC recurrence despite DAA therapy.9 Another study conducted by Cabibbo et al.10 showed that the risk of early HCC recurrence remained high after DAA therapy in patients who achieved complete remission (CR) of HCC. To date, few studies have demonstrated that DAA therapy has a significant beneficial effect in preventing HCC recurrence compared to the untreated or IFN-treated group.11-13 Because of these contradictory results, patients with HCC may be excluded from DAA therapy even after CR is achieved.
- In this context, our study aimed to elucidate the effect of DAA therapy on HCC recurrence risk by comparing patients who received DAA therapy with those who did not in a large-scale HCV-related HCC cohort provided by the Korea Health Insurance Review and Assessment (HIRA) database.
INTRODUCTION
- 1. Patient selection
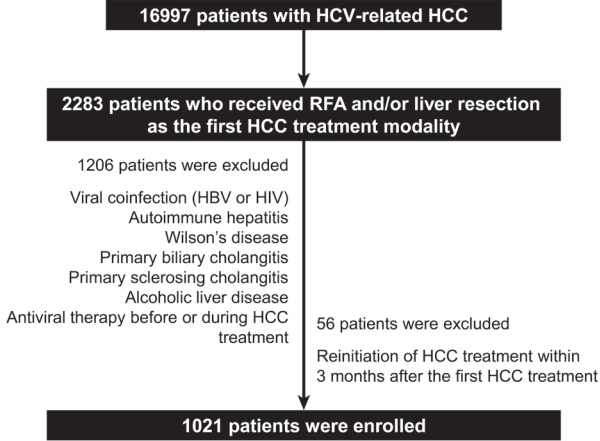
- We retrospectively reviewed patients with HCV-related HCC in the HIRA database. HIRA includes specific and detailed medical information on diagnosis, prescriptions, invasive procedures, surgeries, and sociodemographic data such as the age and sex of patients.14 In South Korea, more than 98% of the total population is enrolled in the National Health Insurance.14 Moreover, all medical institutions are required to practice under the supervision of National Health Insurance, which is based on reimbursement. A total of 2,283 subjects with HCV-related HCC (B18.2 and C22.0) who underwent radiofrequency ablation (RFA; Q7280, Q7281, and QZ841), liver resection (Q7221, Q7222, Q7223, Q7224, Q3051, Q3052, Q3053, Q3054, and Q3055), or both as the first treatment modality for HCC were extracted from the HIRA database using the International Classification of Disease (10th edition). The exclusion criteria were as follows: viral coinfection, including hepatitis B (B18.1, B18.10, B18.18, and Z22.5) and human immunodeficiency virus (B20.X, B21.X, B22.X, B23.X, and B24.X), autoimmune hepatitis (K75.4), Wilson’s disease (E83.0), primary biliary cholangitis (K74.3, K74.30, K74.31, K74.32, and K74.39), primary sclerosing cholangitis (K83.0), alcoholic liver disease (K70.X), and history of antiviral therapy before or during HCC treatment. As radiologic findings were not available in the HIRA database, we excluded 56 patients who received another HCC treatment within 3 months after the first HCC treatment to enroll only patients who achieved complete response or sufficient HCC treatment with the first RFA or liver resection. We finally enlisted 1,021 patients (Fig. 1), who were followed up until death or until December 2016. The Institutional Review Board (IRB) of the Ajou University Hospital approved the study protocol (IRB No. AJIRB-MED-MDB-17-031). Patients were not required to provide informed consent because of the retrospective nature of the study and the use of fully de-identified data. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) reporting guidelines were followed (Supplementary Table 1).
- 2. Definitions
- Data on age, sex, liver cirrhosis (K74.X), diabetes (E11.X, E12.X, E13.X, and E14.X), the first HCC treatment modality, HCC treatment modality after recurrence, duration and regimen of antiviral therapy, and interval between HCC treatment and antiviral therapy were obtained. The antiviral treatment regimens investigated were DAA (638101ATB [daclatasvir], 638001ACH [asunaprevir], 644401ATB [sofosbuvir], 645800ATB [ledipasvir/sofosbuvir]), IFN (175502BIJ, 175503BIJ, 175504BIJ, 175530BIJ, 175601BIJ, 175602BIJ, 175630BIJ, 175631BIJ, 175801BIJ, 175901BIJ, 452602BIJ, 452630BIJ, 454830BIJ, and 454834BIJ), and ribavirin (223604ACH, 223601ACH, and 223601ACH). We assigned patients to the untreated group if HCC recurred before completion of DAA therapy. The primary outcome was HCC recurrence after the first treatment and the secondary outcome was all-cause mortality during follow-up. HCC recurrence was defined as reinitiation of HCC treatment 3 months after the first HCC treatment. The period from the first HCC treatment to HCC recurrence was regarded as the HCC recurrence time. Patients who underwent liver transplantation were treated for HCC recurrence and not censored. Death was defined as the medical outcome classification on the T200 table, and non-hospital deaths were excluded. Diabetes was diagnosed when diagnostic and medication codes were identified simultaneously.
- 3. Statistical analysis
- All data management and analyses were performed using R statistical software (version 3.3.4; R Core Team [2014]; R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/). A P-value <0.05 was considered statistically significant. Pearson’s chi-square test or Fisher’s exact test was used for categorical data; variables with an expected frequency of less than 5 were analyzed using the Fisher’s exact test. Analysis of variance (ANOVA) was used to compare the groups for continuous data. Post-hoc comparisons after ANOVA were controlled using the Bonferroni correction. Factors associated with HCC recurrence or death were identified using the Cox proportional hazards model. In an analysis to ascertain the proportional hazard assumption of DAA and IFN-based treatments, the coefficients of time-dependent variables for treatment modality were identified as insignificant. Therefore, time-varying Cox analysis was not performed in this study.
- We performed landmark analyses at 6 months and 1 year after the first HCC treatment to avoid immortal time bias. Landmark analysis is a suitable method to demonstrate time-to-event as a Kaplan-Meier curve.15 Competing risk analysis was also conducted to correctly estimate the probabilities of HCC recurrence or death in the presence of competing events, such as death or HCC recurrence.16
- The impact of treatment modality on HCC recurrence or all-cause mortality was identified through analysis between the DAA-treated and untreated cohorts and between the antiviral (DAA- or IFN-based)-treated and untreated cohorts.
METHODS
- 1. Baseline characteristics of the HIRA cohort
- A total of 1,021 patients with HCV-related HCC underwent RFA, liver resection, or both as the first treatment modality from January 2007 to December 2016 and did not receive antiviral therapy before HCC treatment. Among them, 77 patients had been treated with DAA therapy, 14 patients had received IFN therapy, and 930 patients did not undergo antiviral therapy after HCC treatment. The mean age and proportion of male patients were 65.6±8.8 years and 44.1% in the DAA-treated group, 58.5±7.5 years and 78.6% in the IFN-treated group, 68.5±8.8 years and 57.7% in the untreated group, respectively. The numbers of patients diagnosed with liver cirrhosis and HCC recurrence were 55 (71.4%) and one (1.3%) in the DAA-treated group, eight (57.1%) and three (21.4%) in the IFN-treated group, and 667 (71.7%) and 507 (54.5%) in the untreated group, respectively. The number of patients who underwent liver resection and RFA was three (3.9%) and 74 (96.1%) in the DAA-treated group, two (14.3%) and 12 (85.7%) in the IFN-treated group, and 102 (11%) and 826 (88.8%) in the untreated group, respectively. Two patients in the untreated group underwent both planned liver resection and RFA during hospitalization. The median DAA therapy duration (interquartile range [IQR]) was 13.1 (8.3-18.7) weeks. Thirty-seven patients received DAA therapy for less than 12 weeks, and 40 patients received DAA therapy for 12-24 weeks. For the IFN-treated group, the median treatment duration (IQR) was 16.6 (5.3-42.6) weeks. Seven patients were treated for less than 12 weeks, five patients for 12-24 weeks, and two patients for more than 24 weeks. The interval between the first HCC treatment to antiviral therapy (IQR) was 14.3 (6.1-39.6) months in the DAA-treated group and 16.1 (2.6-22.1) months in the IFN-treated group (Table 1). The median follow-up period (IQR) was 684 (381-1,294) days in the DAA group, 1,583 (620-2,244) days in the IFN group, and 600 (304-1,163) days in the untreated group. Of the 199 patients who received their first HCC treatment after January 2015, 41 had been treated with DAA therapy, two received IFN therapy, and 156 did not undergo antiviral therapy after HCC treatment. The median follow-up period (IQR) was 394 (275-540) days in the DAA group, 400 (304-496) days in the IFN group, and 306 (148-478) days in the untreated group (Table 2).
- 2. Effect of DAA therapy and antiviral therapy on HCC recurrence
- Using a multivariate Cox proportional hazard regression model, we investigated the impact of antiviral treatment modality on HCC recurrence by analyzing the DAA-treated and untreated cohorts and comparing antiviral (DAA- or IFN-based)-treated and untreated cohorts. The effects of age, sex, diabetes, cirrhosis, and first HCC treatment modality were adjusted for in this analysis. Compared with the untreated cohorts, the DAA-treated cohorts were independently associated with lower HCC recurrence (HR, 0.037; 95% CI, 0.005 to -0.261; P<0.001). In a landmark analysis at 6 months and 1 year after the first HCC treatment, DAA-treated cohorts were consistently associated with lower HCC recurrence rates than untreated cohorts (HR, 0.04; 95% CI, 0.006-0.289; P=0.001; and HR, 0.05; 95% CI, 0.007-0.354; P=0.003, respectively). In an analysis of antiviral (DAA- or IFN-based)-treated and untreated cohorts, antiviral-treated cohorts were independently associated with lower HCC recurrence (HR, 0.086; 95% CI, 0.027-0.268; P<0.001). In a landmark analysis at 6 months and 1 year after the first HCC treatment, antiviral-treated cohorts were also consistently associated with lower HCC recurrence rates than untreated cohorts (HR, 0.094; 95% CI, 0.029-0.293; P<0.001; and HR, 0.111; 95% CI, 0.035-0.346, P<0.001, respectively) (Table 3).
- Fig. 2A shows the cumulative probability of HCC recurrence estimated using the Kaplan-Meier method. The DAA-treated group showed lower probabilities of HCC recurrence than the IFN-treated and untreated groups (log-rank P=0.03, and log-rank P<0.001, respectively). The IFN-treated group was also associated with a lower probability of HCC recurrence than the untreated group (log-rank P=0.015). The cumulative HCC recurrence rates at 1, 2, and 5 years were 0%, 1.8%, and 1.8% in the DAA-treated group; 7.1%, 14.9%, and 14.9% in the IFN-treated group; and 19.7%, 43.6%, and 69.3% in the untreated group, respectively (P<0.001). Among patients with cirrhosis, those who underwent DAA therapy showed lower probabilities of HCC recurrence than the IFN-treated and untreated cohorts (log rank P<0.001 and log rank P=0.03, respectively). The IFN-treated group was also associated with a lower probability of HCC recurrence than the untreated group (log-rank P<0.001). The probabilities of HCC recurrence at 1, 2, and 5 years were 0.0%, 14.3%, and 14.3%, respectively, in the IFN-treated group and 20.7%, 46.1%, and 72.3%, respectively, in the untreated group. In the DAA-treated group, none of the patients with cirrhosis experienced HCC recurrence (P<0.001) (Fig. 2B).
- Supplementary Tables 2, 3 show the effects of other variables on HCC recurrence. According to univariate Cox regression analysis, liver cirrhosis was a significant predictor of HCC recurrence. However, age, sex, diabetes, and first HCC treatment modality, including RFA and surgical resection, were not related to HCC recurrence. Supplementary Tables 4, 5 show the treatment modalities used after the HCC recurrence.
- 3. Impact of DAA therapy and antiviral therapy on all-cause mortality
- We investigated independent factors for all-cause mortality by analyzing the DAA-treated and untreated cohorts and comparing antiviral (DAA- or IFN-based)-treated and untreated cohorts (Table 4). Multivariate regression with the Cox model was adjusted for age, sex, diabetes, cirrhosis, and first HCC treatment modality. DAA-treated cohorts were associated with lower mortality than untreated cohorts (HR, 0.044; 95% CI, 0.006-0.313; P=0.002). In a landmark analysis at 6 months and 1 year after the first HCC treatment, DAA-treated cohorts were associated with lower all-cause mortality than untreated cohorts (HR, 0.049; 95% CI, 0.007-0.349; P =0.003; and HR, 0.063; 95% CI, 0.009-0.451; P=0.006, respectively). In an analysis of antiviral (DAA- or IFN-based)-treated and untreated cohorts, antiviral-treated cohorts were associated with lower all-cause mortality (hazard ratio [HR], 0.066; 95% confidence interval [CI], 0.016-0.266; P<0.001). In a landmark analysis at 6 months and 1 year after the first HCC treatment, antiviral therapy was also consistently associated with lower all-cause mortality than in the untreated cohorts (HR, 0.037; 95% CI, 0.005-0.263; P<0.001; and HR, 0.047; 95% CI, 0.007-0.334; P=0.002, respectively).
RESULTS
- The aim of the present study was to demonstrate the benefits or harms of DAA therapy on HCC recurrence after curative therapy in Korean patients. Our study elucidated that DAA therapy is associated with lower HCC recurrence and all-cause mortality than IFN-based therapy or no treatment in patients with HCV-related HCC after curative therapy.
- Since Reig et al.8 reported an unexpectedly high rate of early HCC recurrence in patients with HCV-related HCC after DAA therapy, many studies have been conducted to refute this observation. Most studies have concluded that DAA therapy does not increase the HCC recurrence rate. However, a serious concern regarding these results is that they amplify rather than resolve suspicions regarding the efficacy of DAA therapy. The findings indicated that DAA therapy did not increase or decrease HCC recurrence. In a study on French ANRS CO22 HEPATHER cohorts, including 189 patients who underwent DAA therapy and 78 patients who did not receive antiviral therapy, the HCC recurrence rates were 0.73/100 person-months and 0.66/100 person-months in the DAA-treated and untreated groups, respectively (P=0.8756).17 Singal et al.18 also demonstrated that DAA therapy was not related to increased or decreased HCC recurrence rate using landmark analysis at 90 days (HR, 0.92; 95% CI, 0.60-1.41), 120 days (HR, 0.99; 95% CI, 0.66-1.48), and 6 months (HR, 0.98; 95% CI, 0.69-1.40) from CR. Another study showed that the cumulative probabilities of HCC recurrence-free survival at 1 and 2 years were 85.1% and 73.2% in the DAA-treated group and 82.9% and 78.2% in the untreated group (P=0.278), respectively.19 Moreover, some studies have suggested that DAA therapy is inferior to IFN therapy in the inhibition of HCC recurrence. IFN-based treatment has previously been shown to be effective for recurrence-free survival.20-22 In 2020, Kuo et al.23 reported that the DAA-treated group showed poor HCC recurrence-free survival compared to the IFN-treated group (75.4% vs. 95.0%, P<0.001). Another study demonstrated that the tertiary prevention effect on HCC recurrence was invalid in DAA therapy but was durable in IFN-based therapy.24 Most of the authors who insisted that DAA therapy increases HCC recurrence or that DAA therapy is inferior to IFN therapy for secondary prevention of HCC present immunological hypotheses. In 2015, Serti et al.25 reported that DAA-induced rapid HCV clearance was related to a decline in intrahepatic immune function mediated by IFN and natural killer cell activation. It has been hypothesized that these immune-mediated inhibitory effects on HCC cell proliferation change after DAA therapy. These changes are also associated with HCC recurrence.21 In contrast, Minami et al.12 reported that DAA therapy was not inferior to IFN treatment for the prevention of early HCC recurrence (HR, 0.65; P=0.28). Nagata et al.11 also revealed that the early HCC recurrence risk was similar between the IFN-treated and DAA-treated groups (P=0.54). A meta-regression conducted by Waziry et al.26 also showed that DAA therapy was not related to higher HCC recurrence than IFN treatment (adjusted rate ratio, 0.62; 95% CI, 0.11-3.45; P=0.56).
- An ostensible consensus on DAA therapy and HCC recurrence seems to have already been reached. However, there has been an active debate over this controversial topic. In 2018, the European Association for the Study of the Liver guidelines also stated that it is unclear whether DAA therapy increases the HCC recurrence rate and suggested close surveillance in these patients.27 According to the 2018 American Association for the Study of Liver Diseases guidelines for HCC management, the impact of DAA therapy on the potential risk of HCC recurrence is uncertain and requires further investigation.28 As no medical provider will prescribe medications without a therapeutic effect, it is necessary to continue to prove through further research that DAA therapy is at least equivalent to IFN-based therapy and superior to the untreated group in inhibiting HCC recurrence.
- Although studies on DAA therapy-related HCC recurrence have been actively conducted worldwide, large-scale population-based investigations in Korean cohorts have not been conducted. In the present study, we included over 1,000 Korean patients with an acceptable follow-up period. Our study, which included patients without antiviral therapy as a control group, demonstrated that DAA therapy is an independent protective factor against HCC recurrence and all-cause mortality in patients with HCV-related HCC after curative treatment. In addition, Kaplan-Meier analysis using the log-rank test with Bonferroni corrections showed that DAA therapy is more effective in preventing HCC recurrence than IFN-based therapy.
- Our study had several limitations. First, the limited number of study subjects in the IFN and DAA groups did not allow for a definite conclusion within this study. Furthermore, approximately 50% of the IFN-treated group received insufficient treatment duration, and the effect of IFN treatment on HCC recurrence could not be adequately reflected. Second, because data were extracted from HIRA, the results of imaging studies and laboratory findings such as alpha-fetoprotein, total bilirubin, international normalized ratio, and albumin were not available. Thus, the detailed tumor burden, underlying liver function, and CR acquisition status based on radiologic findings could not be identified. Furthermore, we could not perform PS matching because of data limitations. Third, because HCC recurrence was defined according to the reinitiation of HCC treatment, patients who stopped treatment when HCC recurred after the first treatment may have been misclassified as the non-recurrence group. We struggled to establish inclusion criteria because of these limitations. We are convinced that these controversies have been overcome by including only those patients who received RFA or underwent liver resection as an initial HCC treatment and did not undergo any other treatment within 3 months. Fourth, we only presented the results from landmark analysis as a table, not a figure. This can be misleading to the readers. However, we presented Kaplan-Meier curves because the group variable did not violate the assumption with testing by the Schoenfeld residuals tests (IFN group rho=-0.0302. chisq=0.467, P=0.494/DAA group rho=-0.0267, chisq=0.365, P=0.546).29
- In conclusion, DAA therapy was not associated with an increase in the HCC recurrence rate after RFA or liver resection but was associated with a significantly lower HCC recurrence rate than the untreated group. Therefore, clinicians should consider administering DAA therapy after curative HCC treatment in patients with HCV-related HCC.
DISCUSSION
-
Conflicts of Interest
The authors have no conflicts to disclose.
-
Ethics Statement
Patients were not required to provide informed consent because of the retrospective nature of the study and the use of fully de-identified data. The Institutional Review Board (IRB) of the Ajou University Hospital approved the study protocol (IRB No. AJIRB-MED-MDB-17-031).
-
Funding Statement
This research was supported by the Research Award of the Korean Liver Cancer Association (2021), the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea (HI19C0872 and HR21C1003), and the Bio and Medical Technology Development Program of the National Research Foundation (NRF-2018M3A9E8023861 and NRF-2019R1C1C1004580) funded by the Korean government (MSIT).
-
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.
-
Author Contribution
Conceptualization: Kim SS, Ahn Y-H
Data curation: Cheong JY, Cho HJ, Lee H, Han JE
Formal analysis: Kim SS, Park B, Lee H, Ahn Y-H
Funding acquisition: Kim SS, Cheong JY
Investigation: Cheong JY, Cho HJ, Ahn Y-H, Han JE
Methodology: Kim SS, Park B
Project admission: Kim SS, Park B
Resource: Ahn Y-H, Lee H, Kim SS, Park B
Software: Park B, Lee H
Supervision: Kim SS, Park B
Validation: Park B, Lee H
Visualization: Lee H, Han JE
Writing-Original draft preparation: Ahn Y-H
Writing-Review and editing: Kim SS, Park B
Article information
Supplementary Material

| Variable | DAA-treated group (n=77) | IFN-treated group (n=14) | Untreated group (n=930) | P-value |
|---|---|---|---|---|
| Age (years) | 65.6±8.8 | 58.5±7.5 | 68.5±8.8 | <0.001* |
| Male gender | 34 (41.2) | 11 (78.6) | 537 (57.7) | 0.017* |
| Diabetes mellitus | 35 (45.5) | 7 (50.0) | 341 (36.7) | 0.193 |
| Liver cirrhosis | 55 (71.4) | 8 (57.1) | 667 (71.7) | 0.457 |
| HCC recurrence | 1 (1.3) | 3 (21.4) | 507 (54.5) | <0.001* |
| HCC treatment modality | 0.196 | |||
| Resection | 3 (3.9) | 2 (14.3) | 102 (11.0) | |
| RFA | 74 (96.1) | 12 (85.7) | 826 (88.8) | |
| Resection+RFA | 2 (0.2) | |||
| DAA regimen | ||||
| Daclatasvir/asunaprevir | 46 (59.7) | |||
| Sofosbuvir/ribavirin | 25 (32.5) | |||
| Ledipasvir/sofosbuvir | 6 (7.8) | |||
| DAA regimen duration (weeks) | 13.1 (8.3-18.7) | |||
| <12 | 37 (48.1) | |||
| 12-24 | 40 (51.9) | |||
| IFN regimen duration (weeks) | 16.6 (5.3-42.6) | |||
| <12 | 7 (50.0) | |||
| 12-24 | 5 (35.7) | |||
| >24 | 2 (14.3) | |||
| Interval between HCC treatment to antiviral therapy, months | 14.3 (6.1-39.6) | 16.1 (2.6-22.1) | ||
| <3 | 12 (15.6) | 4 (28.6) | ||
| 3-6 | 7 (9.1) | 2 (14.3) | ||
| 6-12 | 15 (19.5) | 2 (14.3) | ||
| 12-24 | 16 (20.7) | 3 (21.4) | ||
| >24 | 27 (35.1) | 3 (21.4) |
| Variable | DAA-treated group (n=41) | IFN-treated group (n=2) | Untreated group (n=156) | P-value |
|---|---|---|---|---|
| Age (years) | 66.1±8.6 | 55.0±9.9 | 70.2±9.1 | 0.003* |
| Male gender | 18 (43.9) | 1 (50.0) | 88 (56.4) | 0.318 |
| Diabetes mellitus | 21 (51.2) | 1 (50.0) | 63 (40.4) | 0.413 |
| Liver cirrhosis | 30 (73.2) | 1 (50.0) | 117 (75.0) | 0.62 |
| HCC recurrence | 1 (2.4) | 0 (0.0) | 30 (19.2) | 0.013* |
| HCC treatment modality | 1 | |||
| Resection | 3 (1.9) | |||
| RFA | 41 (100.0) | 2 (100.0) | 153 (98.1) | |
| DAA regimen | ||||
| Daclatasvir/asunaprevir | 24 (58.5) | |||
| Sofosbuvir/ribavirin | 12 (29.3) | |||
| Ledipasvir/sofosbuvir | 5 (12.2) | |||
| DAA regimen duration (weeks) | 16.1 (13.1-21.1) | |||
| <12 | 14 (34.1) | |||
| 12-24 | 27 (65.9) | |||
| IFN regimen duration (weeks) | 10.3 (7.8-10.2) | |||
| <12 | 2 (100.0) | |||
| Interval between HCC treatment to antiviral therapy, months | 6.4 (2.9-11.5) | 6.1 (5.8-6.4) | ||
| <3 | 12 (29.3) | |||
| 3-6 | 7 (17.1) | 1 (50.0) | ||
| 6-12 | 13 (31.7) | 1 (50.0) | ||
| 12-24 | 9 (21.9) |
| Modality |
Univariate Cox |
Multivariate Cox* |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| DAA vs. untreated | 0.039 | 0.005-0.274 | 0.001† | 0.037 | 0.005-0.261 | <0.001† |
| DAA‡ vs. untreated | 0.042 | 0.006-0.303 | 0.001† | 0.040 | 0.006-0.289 | 0.001† |
| DAA§ vs. untreated | 0.052 | 0.007-0.371 | 0.003† | 0.050 | 0.007-0.354 | 0.003† |
| DAA or IFN vs. untreated | 0.089 | 0.029-0.279 | <0.001† | 0.086 | 0.027-0.268 | <0.001† |
| DAA or IFN‡ vs. untreated | 0.098 | 0.032-0.307 | <0.001† | 0.094 | 0.029-0.293 | <0.001† |
| DAA or IFN§ vs. untreated | 0.117 | 0.038-0.368 | <0.001† | 0.111 | 0.035-0.346 | <0.001† |
HR, hazard ratio; CI, confidence interval; CHC, chronic hepatitis C; HCC, hepatocellular carcinoma; DAA, direct acting antiviral agent; IFN, interferon.
* Adjusted for sex, age, diabetes, cirrhosis, first HCC treatment modality;
† P<0.05;
‡ Landmark analysis at 6 months after first HCC treatment;
§ Landmark analysis at 1 year after first HCC treatment.
| Modality |
Univariate Cox |
Multivariate Cox* |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| DAA vs. untreated | 0.042 | 0.006-0.297 | 0.002† | 0.044 | 0.006-0.313 | 0.002† |
| DAA‡ vs. untreated | 0.047 | 0.007-0.336 | 0.002† | 0.049 | 0.007-0.349 | 0.003† |
| DAA§ vs. untreated | 0.060 | 0.008-0.427 | 0.004† | 0.063 | 0.009-0.451 | 0.006† |
| DAA or IFN vs. untreated | 0.060 | 0.015-0.243 | <0.001† | 0.066 | 0.016-0.266 | <0.001† |
| DAA or IFN‡ vs. untreated | 0.034 | 0.005-0.241 | <0.001† | 0.037 | 0.005-0.263 | <0.001† |
| DAA or IFN§ vs. untreated | 0.042 | 0.006-0.300 | 0.002† | 0.047 | 0.007-0.334 | 0.002† |
HR, hazard ratio; CI, confidence interval; CHC, chronic hepatitis C; DAA, direct acting antiviral agent; IFN, interferon.
* Adjusted for sex, age, diabetes, cirrhosis, first HCC treatment modality;
† P<0.05;
‡ Landmark analysis at 6 months after first HCC treatment;
§ Landmark analysis at 1 year after first HCC treatment.
- 1. Zeuzem S, Feinman SV, Rasenack J, Heathcote EJ, Lai MY, Gane E, et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med 2000;343:1666−1672.ArticlePubMed
- 2. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958−965.ArticlePubMed
- 3. Lawitz E, Makara M, Akarca US, Thuluvath PJ, Preotescu LL, Varunok P, et al. Efficacy and safety of ombitasvir, paritaprevir, and ritonavir in an open-label study of patients with genotype 1b chronic hepatitis C virus infection with and without cirrhosis. Gastroenterology 2015;149:971−980. e1.ArticlePubMed
- 4. Cho BW, Kim SB, Song IH, Lee SH, Kim HS, Lee TH, et al. Efficacy and safety of daclatasvir plus asunaprevir for Korean patients with HCV genotype Ib infection: a retrospective multi-institutional study. Clin Mol Hepatol 2017;23:51−56.ArticlePubMedPMCPDF
- 5. World Health Organization (WHO). Global hepatitis report, 2017 [Internet]. Geneva: WHO; [cited 2019 Jan 1]. Available from: https://www.who.int/publications/i/item/9789241565455
- 6. Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017;2:161−176.PubMed
- 7. Hanouneh IA, Alkhouri N, Singal AG. Hepatocellular carcinoma surveillance in the 21st century: saving lives or causing harm? Clin Mol Hepatol 2019;25:264−269.ArticlePubMedPMCPDF
- 8. Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol 2016;65:719−726.ArticlePubMed
- 9. Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol 2016;65:727−733.ArticlePubMed
- 10. Cabibbo G, Petta S, Calvaruso V, Cacciola I, Cannavò MR, Madonia S, et al. Is early recurrence of hepatocellular carcinoma in HCV cirrhotic patients affected by treatment with direct-acting antivirals? A prospective multicentre study. Aliment Pharmacol Ther 2017;46:688−695.ArticlePubMedPDF
- 11. Nagata H, Nakagawa M, Asahina Y, Sato A, Asano Y, Tsunoda T, et al. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J Hepatol 2017;67:933−939.ArticlePubMed
- 12. Minami T, Tateishi R, Nakagomi R, Fujiwara N, Sato M, Enooku K, et al. The impact of direct-acting antivirals on early tumor recurrence after radiofrequency ablation in hepatitis C-related hepatocellular carcinoma. J Hepatol 2016;65:1272−1273.ArticlePubMed
- 13. Nagaoki Y, Imamura M, Nishida Y, Daijo K, Teraoka Y, Honda F, et al. The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma: comparison with interferon-based therapy. J Med Virol 2019;91:650−658.ArticlePubMedPDF
- 14. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci 2017;32:718−728.ArticlePubMedPMCPDF
- 15. Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol 2013;31:2963−2969.ArticlePubMedPMC
- 16. Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999;18:695−706.ArticlePubMed
- 17. ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: data from three ANRS cohorts. J Hepatol 2016;65:734−740.ArticlePubMed
- 18. Singal AG, Rich NE, Mehta N, Branch A, Pillai A, Hoteit M, et al. Direct-acting antiviral therapy not associated with recurrence of hepatocellular carcinoma in a multicenter North American cohort study. Gastroenterology 2019;156:1683−1692. e1.ArticlePubMedPMC
- 19. Lin WC, Lin YS, Chang CW, Chang CW, Wang TE, Wang HY, et al. Impact of direct-acting antiviral therapy for hepatitis C-related hepatocellular carcinoma. PLoS One 2020;15:e0233212.ArticlePubMedPMC
- 20. Shen YC, Hsu C, Chen LT, Cheng CC, Hu FC, Cheng AL. Adjuvant interferon therapy after curative therapy for hepatocellular carcinoma (HCC): a meta-regression approach. J Hepatol 2010;52:889−894.ArticlePubMed
- 21. Hsu CS, Chao YC, Lin HH, Chen DS, Kao JH. Systematic review: impact of interferon-based therapy on HCV-related hepatocellular carcinoma. Sci Rep 2015;5:9954. ArticlePubMedPMCPDF
- 22. Kusano H, Akiba J, Ogasawara S, Sanada S, Yasumoto M, Nakayama M, et al. Pegylated interferon-α2a inhibits proliferation of human liver cancer cells in vitro and in vivo. PLoS One 2013;8:e83195.ArticlePubMedPMC
- 23. Kuo YH, Wang JH, Chang KC, Hung CH, Lu SN, Hu TH, et al. The influence of direct-acting antivirals in hepatitis C virus related hepatocellular carcinoma after curative treatment. Invest New Drugs 2020;38:202−210.ArticlePubMedPDF
- 24. Teng W, Jeng WJ, Yang HI, Chen WT, Hsieh YC, Huang CH, et al. Interferon is superior to direct acting antiviral therapy in tertiary prevention of early recurrence of hepatocellular carcinoma. Cancers (Basel) 2019;12:23. ArticlePubMedPMC
- 25. Serti E, Chepa-Lotrea X, Kim YJ, Keane M, Fryzek N, Liang TJ, et al. Successful interferon-free therapy of chronic hepatitis C Virus infection normalizes natural killer cell function. Gastroenterology 2015;149:190−200. e2.ArticlePubMedPMC
- 26. Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J Hepatol 2017;67:1204−1212.ArticlePubMed
- 27. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182−236.ArticlePubMed
- 28. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358−380.ArticlePubMedPDF
- 29. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515−526.Article
References
Figure & Data
References
Citations

- Comparison of Surgical Resection and Radiofrequency Ablation in Elderly Patients with Hepatocellular Carcinoma
Jun Il Kim, Jayoun Lee, Gi Hong Choi, Min Woo Lee, Dong Ah Park, Jeong-Ju Yoo
Digestive Diseases and Sciences.2024; 69(3): 1055. CrossRef - Analyzing risk factors and developing a stratification system for hepatocellular carcinoma recurrence after interferon-free direct-acting antiviral therapy in chronic hepatitis C patients
Chih-Hsuan Luan, Pin-Shuo Su, Chi-Jen Chu, Chung-Chi Lin, Chien-Wei Su, Jiing-Chyuan Luo, I-Cheng Lee, Chen-Ta Chi, Shou-Dong Lee, Yuan-Jen Wang, Fa-Yauh Lee, Yi-Hsiang Huang, Ming-Chih Hou
Journal of the Chinese Medical Association.2024; 87(4): 357. CrossRef - Addition of Kidney Dysfunction Type to MELD-Na for the Prediction of Survival in Cirrhotic Patients Awaiting Liver Transplantation in Comparison with MELD 3.0 with Albumin
Kyeong-Min Yeom, Jong-In Chang, Jeong-Ju Yoo, Ji Eun Moon, Dong Hyun Sinn, Young Seok Kim, Sang Gyune Kim
Diagnostics.2023; 14(1): 39. CrossRef - Is direct-acting antiviral treatment beneficial or harmful for patients with hepatitis C virus-related hepatocellular carcinoma?
Hye Won Lee
Journal of Liver Cancer.2022; 22(2): 91. CrossRef
 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation
- Download Citation
- Close
- Related articles
-
- The diagnostic value of circulating tumor DNA in hepatitis B virus induced hepatocellular carcinoma: a systematic review and meta-analysis
- Is direct-acting antiviral treatment beneficial or harmful for patients with hepatitis C virus-related hepatocellular carcinoma?
- Stereotactic body radiation therapy for elderly patients with small hepatocellular carcinoma: a retrospective observational study
- Systemic therapy for advanced hepatocellular carcinoma: consideration for selecting second-line treatment

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION


 Follow JLC on Twitter
Follow JLC on Twitter