Articles
- Page Path
- HOME > J Liver Cancer > Volume 23(1); 2023 > Article
-
Review Article
Radiologic features of hepatocellular carcinoma related to prognosis -
Shin Hye Hwang1
 , Hyungjin Rhee2,3,4
, Hyungjin Rhee2,3,4
-
Journal of Liver Cancer 2023;23(1):143-156.
DOI: https://doi.org/10.17998/jlc.2023.02.16
Published online: March 9, 2023
1Department of Radiology, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea
2Department of Radiology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
3Research Institute of Radiological Science, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
4Center for Clinical Imaging Data Science, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
-
Corresponding author: Hyungjin Rhee, Department of Radiology, Severance Hospital, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea
Tel. +82-2-2228-2353, Fax. +82-2-2227-8337 E-mail: hjinrhee@yuhs.ac
© 2023 The Korean Liver Cancer Association.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
- 2,030 Views
- 111 Downloads
- 2 Citations
- Abstract
- INTRODUCTION
- FAT IN MASS
- IMAGING FINDINGS OF ARTERIAL PHASE
- ENHANCING CAPSULE APPEARANCE ON PORTAL, DELAYED, OR TRANSITIONAL PHASES
- IMAGING FINDINGS OF HEPATOBILIARY PHASE
- LOW APPARENT DIFFUSION COEFFICIENT ON DIFFUSION-WEIGHTED IMAGE
- LR-M CATEGORY OF LI-RADS
- CURRENT LIMITATIONS AND THE NEED FOR FUTURE RESEARCH
- CONCLUSION
- Article information
- References
Abstract
- The cross-sectional imaging findings play a crucial role in the diagnosis of hepatocellular carcinoma (HCC). Recent studies have shown that imaging findings of HCC are not only relevant for the diagnosis of HCC, but also for identifying genetic and pathologic characteristics and determining prognosis. Imaging findings such as rim arterial phase hyperenhancement, arterial phase peritumoral hyperenhancement, hepatobiliary phase peritumoral hypointensity, non-smooth tumor margin, low apparent diffusion coefficient, and the LR-M category of the Liver Imaging-Reporting and Data System have been reported to be associated with poor prognosis. In contrast, imaging findings such as enhancing capsule appearance, hepatobiliary phase hyperintensity, and fat in mass have been reported to be associated with a favorable prognosis. Most of these imaging findings were examined in retrospective, single-center studies that were not adequately validated. However, the imaging findings can be applied for deciding the treatment strategy for HCC, if their significance can be confirmed by a large multicenter study. In this literature, we would like to review imaging findings related to the prognosis of HCC as well as their associated clinicopathological characteristics.
- Hepatocellular carcinoma (HCC) is the most common primary liver cancer, frequently occurring in patients with chronic liver disease or cirrhosis. HCC is characterized as a heterogeneous tumor considering its genetic and pathologic features and prognosis. The well-known prognostic factors of HCC include serum markers such as serum levels of α-fetoprotein (AFP) and protein induced by vitamin K absence or antagonists-II (PIVKA-II), pathologic features such as differentiation, microvascular invasion, satellitosis, subtype, immunohistochemical expression of keratin 19 (K19), and genetic features such as fibroblast growth factor 19 amplification and proliferative class.1 Imaging findings representing the extent of tumor, including tumor size, number, gross vascular invasion, and extrahepatic metastasis, have also been recognized as prognostic factors and incorporated into current staging systems.2-4
- Cross-sectional imaging findings play a crucial role in the diagnosis of HCC. Liver magnetic resonance imaging (MRI) protocols commonly consist of T2-weighted images, dual gradient-echo images to assess the presence of fat and iron, fat-suppressed T1-weighted dynamic enhancement images to characterize dynamic enhancement patterns, and diffusion-weighted images to assess the free diffusion of water molecules, which are known to be related to tumor cellularity or necrosis. Hepatobiliary phase images are added when gadoxetic acid (Gd-EOB-DTPA, Bayer HealthCare Pharmaceuticals, Berlin, Germany) is used as the contrast medium. Particularly, imaging findings in dynamic phases employing intravenous contrast agents are most important for HCC imaging diagnosis, since they reveal characteristic vascular changes during multistep hepatocarcinogenesis. In addition, the hepatobiliary phase is another key sequence that shows the difference between HCC and non-tumor livers. The uptake of gadoxetic acid by normal hepatocytes occurs via organic anion transporter polypeptide 8 (OATP8); the period when this uptake is most evident is called the hepatobiliary phase, which corresponds to approximately 15–20 minutes after intravenous injection of gadoxetic acid. Since the expression of OATP8 and the hepatobiliary uptake of gadoxetic acid decline progressively during multistep hepatocarcinogenesis, HCC exhibits a lower hepatobiliary uptake and signal intensity than non-tumor liver.5
- The contemporary guidelines of HCC recommend imaging-only diagnosis of HCC without pathological confirmation when a hepatic lesion shows typical imaging findings of HCC in individuals at a high risk of HCC.6-8 Since several HCC cases are diagnosed based on imaging findings, without obtaining tissue, it is difficult to apply genetic and pathologic prognostic factors in such cases. Recent studies have shown that imaging findings of HCC are not only relevant for the diagnosis of HCC, but also for identifying genetic and pathologic characteristics and determining the prognosis (Table 1).9-12
- Here, we review the imaging features of HCC associated with the prognosis of HCC patients.
INTRODUCTION
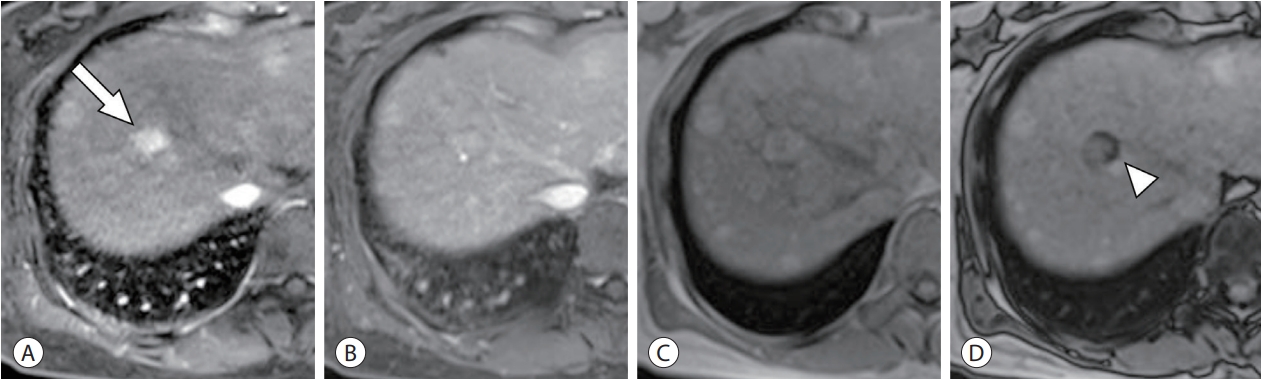
- Fat in mass is one of the Liver Imaging Reporting and Data System (LI-RADS) ancillary features favoring HCC in particular.13 The loss of signal intensity on T1-weighted out-ofphase images compared with in-phase images of dual gradient-echo sequences suggests the presence of fat.14 Because fat is rarely found in pure cholangiocarcinoma, the presence of intralesional fat is useful for the differential diagnosis between hepatocellular lesions and cholangiocarcinoma.15 The mechanism by which fat develops inside HCC is presumed to be clonal expansion of hepatocytes with anomalous fat metabolism and cellular metabolic disturbances due to switching of the dominant blood supply from portal venous to hepatic arterial and the consequent ischemic/hypoxic conditions.16,17 It is most often observed in early HCC <1.5 cm in diameter and tends to decrease with increasing tumor size and grade (Fig. 1).17 However, in steatohepatitic HCC, which is most commonly found in background steatohepatitis or non-alcoholic fatty liver disease, intralesional fat can be observed not only in early HCC but also in advanced tumor grades.18-20
- It has been reported that radiological or histological fat in mass is associated with infrequent microvascular invasion (MVI), suggesting that fat in mass may be associated with a better prognosis.18,21,22 Indeed, several studies have reported an association between fat in mass and a favorable prognosis after curative treatment. Siripongsakun et al.23 reported fewer distant metastases (4.3% vs. 21.7%) and longer time to progression in fat-containing HCCs than in non-fat-containing HCCs in a case-control study of patients who received local treatment or liver transplantation. A study conducted by Chen et al.24 showed that fat in mass was a favorable prognostic factor for tumor recurrence in patients with LR-5 HCCs who underwent hepatic resection. Another study suggested that fat in mass was a favorable prognostic factor for patients undergoing radiofrequency ablation.25
FAT IN MASS
- During multistep hepatocarcinogenesis, HCC becomes hypervascular with the development of unpaired arteries and sinusoidal capillarization.26 Arterial phase hyperenhancement (APHE) is one of the most important imaging findings in HCC diagnosis. Typically, HCC shows APHE of non-rim pattern; LI-RADS defines it as “non-rim like enhancement in arterial phase unequivocally greater in whole or in part than liver.”13
- 1. Rim arterial phase hyperenhancement
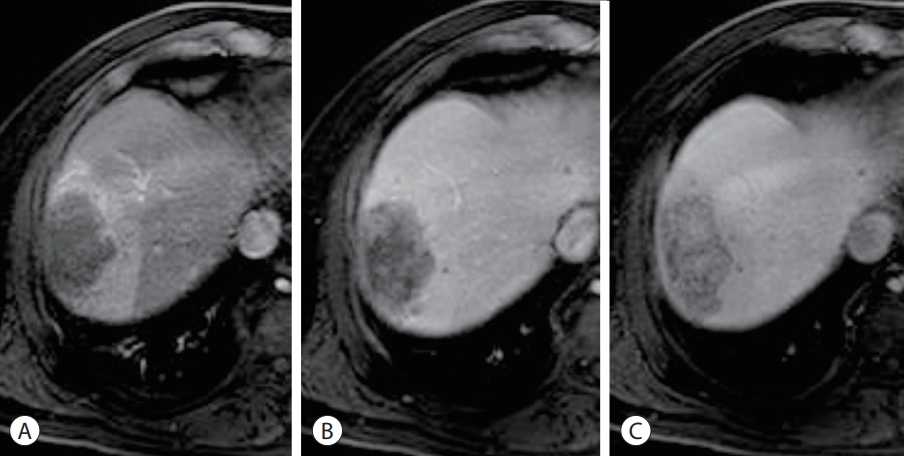
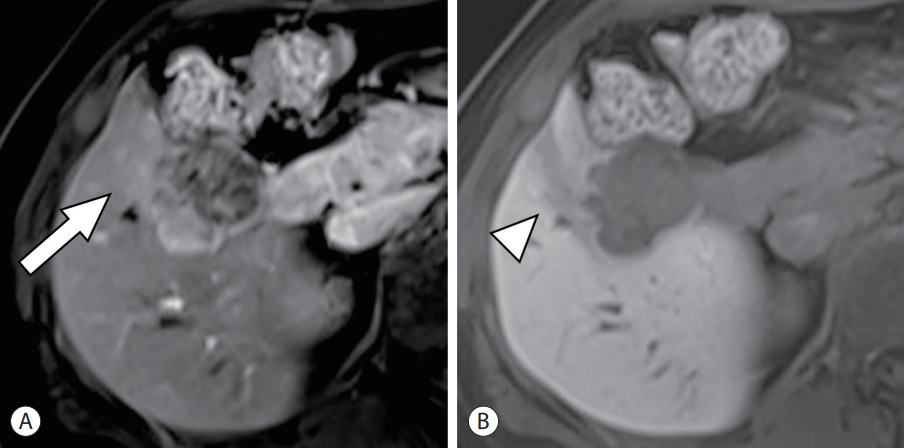
- Rim-APHE is defined as a “spatially defined subtype of APHE in which arterial phase enhancement is most pronounced in the observation periphery” (Figs. 2-4).13 Rim-APHE is more commonly seen in intrahepatic cholangiocarcinoma and combined hepatocellular and cholangiocarcinoma, and is uncommon (5.6–15.7%) in HCC.27-30 It should be distinguished from the “enhancing capsule” and “arterial phase peritumoral enhancement,” as described below.
- HCCs with rim-APHE have more prominent hypoxic and fibrotic tumor microenvironments. HCC with rim-APHE in gadoxetic acid-enhanced MRI was associated with a larger proportion of necrotic area and fibrous stroma, frequent expression of hypoxia-related markers (carbonic anhydrase IX) and stem/progenitor markers (K19 or epithelial cell adhesion molecule [EpCAM]), and histopathologic macrotrabecular pattern.11,30 HCC with rim-APHE shows a characteristic vascular phenotype, including lower microvascular density and a sinusoid-like microvascular pattern.30 The sinusoid-like microvascular pattern, otherwise known as vessels that encapsulate the tumor cluster (VETC) pattern, is known to be associated with frequent microvascular invasion, metastasis, and poor prognosis.31,32 HCC with rim-APHE is also associated with peculiar genetic characteristics, including TP53 mutations, cholangiocarcinoma-like, and proliferative gene expression.9,11,33
- Rim-APHE of HCC is reported to be related to MVI,34 rapid tumor growth,35 frequent early recurrence, poor disease-free survival, poor overall survival, and an increased incidence of extrahepatic metastasis after curative resection or radiofrequency ablation.11,28,29,36 It has also been reported that patients with HCCs showing rim-APHE have a high non-responder rate and low overall survival after chemoembolization.37
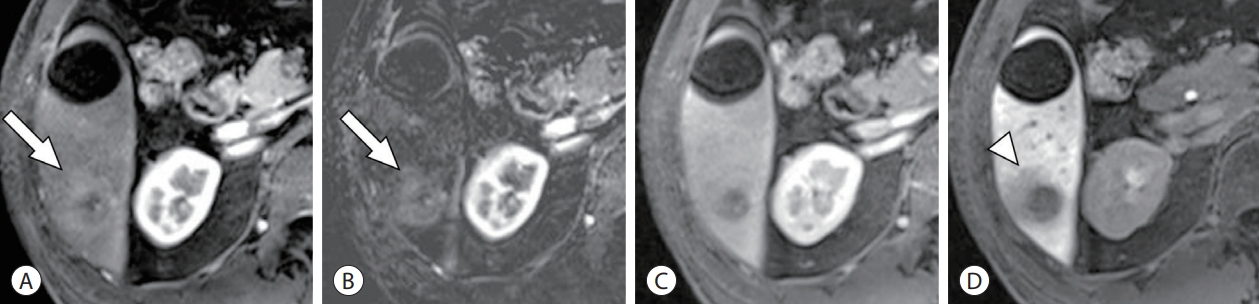
- 2. Arterial phase peritumoral hyperenhancement
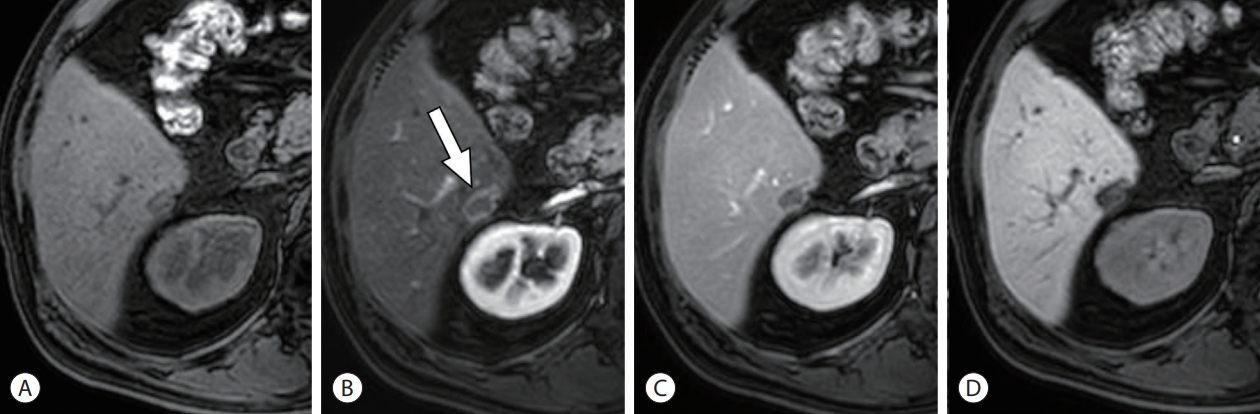
- Arterial phase peritumoral hyperenhancement refers to “early arterial phase wedge-shaped or irregular and circumferential enhancement in parenchyma adjacent to the tumor that fades during later phase” (Figs. 4-6).13,38 It is thought to be compensatory arterial hyperperfusion due to decreased portal venous flow resulting from obstruction of minute portal vein branches around the tumor by microscopic tumor thrombi.39 A few studies have reported it as a predictor of MVI27,40-43 and early recurrence after curative resection.28,44-46
- Arterial phase peritumoral hyperenhancement may resemble corona enhancement and it is therefore vital to differentiate between them.13,38 Both imaging findings are observed in the peritumoral area during the late arterial phase. The arterial phase peritumoral hyperenhancement is caused by compensatory arterial blood flow; therefore, it appears in the early arterial phase and then fades out.39 It often has a geographic or wedge-shaped boundary, with a straight border representing the vascular territory, and can be extensive. Corona enhancement is thought to be venous drainage of hypervascular HCC; therefore, it is observed in the late arterial and early portal phases, then fades out, looks circumferential or eccentric, and is rarely extensive. Corona enhancement is an imaging finding of progressed HCC rather than early HCC, as it appears in the process of venous drainage change from intralesional hepatic vein to peritumoral sinusoid and portal vein at the later stage of hepatocarcinogenesis.47 The area of corona enhancement might be the first site of micrometastasis,39 and corona enhancement has been reported as a predictor of MVI43 and early recurrence after surgery.44-46
- However, as arterial peritumoral enhancement and corona enhancement often appear similar and multiple arterial phases with a high temporal resolution are required for accurate differentiation between them, studies have thus far not attempted to assess their prognostic value separately due to technical reasons. With recent advances in MRI technology, the impact of corona enhancement on prognosis may be reevaluated in the future.
IMAGING FINDINGS OF ARTERIAL PHASE
- The pathological fibrotic capsule is considered to be the result of compression of peritumoral fibrous tissue by the tumor and is closely related to the gross morphology of HCC.48,49 HCCs showing a vaguely nodular type, mostly early HCCs, do not show a fibrotic capsule because of lepidic growth. Fibrotic capsules are mainly observed in nodular HCCs, including HCCs of the single nodular type, single nodular with extranodular growth type, and multinodular confluent type. The fibrotic capsule temporarily serves as a physical barrier to block tumor infiltration; however, capsular and extracapsular tumor infiltration emerge as HCC progresses, and eventually the capsule is seldom observed in the infiltrative type of HCC.48,50 Collectively, the pathological fibrous capsule is not commonly observed; in early HCC it shows vaguely nodular margins and in advanced HCC it shows infiltrative margins.
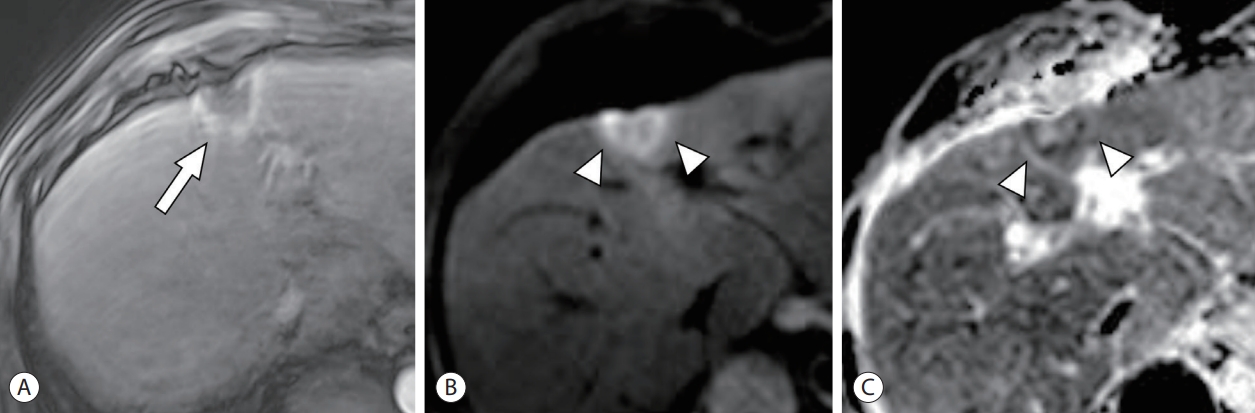
- “Enhancing capsule” is one of the major imaging features for diagnosis of HCC in LI-RADS. It refers to the observation of a smooth, uniform, sharp border around most or all of an observation in the portal venous phase, delayed phase, or transitional phase (Fig. 7).13 The fibrous capsule is strongly associated with enhancing capsule appearance although this is not always the case. Approximately 14–17% of the enhancing capsules are not pathologically fibrous capsules, but pseudo-capsules consisting of prominent sinusoids and peritumoral fibrosis mimicking bridging fibrosis.51,52
- Some studies have indicated that the presence of an enhancing capsule on imaging may be a good prognostic factor in patients undergoing hepatic resection or transarterial chemoembolization.53-55 However, other studies have shown that the enhanced capsule is not significant for prognosis or related to a worse prognosis.27,56 The inconsistent results regarding the prognostic significance of the pathologic or radiologic capsule in literature may be attributed to the heterogeneity of the lesions included in each study.
ENHANCING CAPSULE APPEARANCE ON PORTAL, DELAYED, OR TRANSITIONAL PHASES
- In multistep hepatocarcinogenesis, OATP8 expression gradually decreases, resulting in a decrease in hepatobiliary phase signal intensity on MRI, and these changes are known to precede the formation of unpaired arteries.57 Therefore, hepatobiliary phase hypointensity is a useful imaging finding for malignant transformation of hepatic nodules. In contrast, hepatobiliary phase isointensity represents the normal functioning of hepatocytes and biliary drainage inside the lesion, which generally suggests benignity.13
- 1. Hepatobiliary phase hyperintensity
- Approximately 9–15% of HCC show hyperintensity in the hepatobiliary phase due to OATP8 overexpression,58,59 which is related to the activation of the Wnt/β-catenin pathway and/or hepatocyte nuclear factor 4-α pathway (Fig. 7).60 They should be differentiated from focal nodular hyperplasia and hepatocellular adenomas with β-catenin mutation.61,62
- Most (approximately 80%) hepatobiliary phase hyperintense HCCs were moderately differentiated, while some were well differentiated and were not observed in poorly differentiated HCCs.63 In addition, these HCCs have less frequent MVI, lower serum levels of AFP and PIVKA-II, and decreased immunohistochemical expression of AFP, EpCAM, and glypican 3.58,63,64 Hepatobiliary hyperintense HCC tends to be associated with favorable prognosis after hepatic resection.59,65
- 2. Hepatobiliary phase peritumoral hypointensity
- Peritumoral hypointensity in the hepatobiliary phase refers to a “wedge-shaped or flame-like hypointense area of hepatic parenchyma located outside of the tumor margin” (Figs. 4-6).66 It is not currently included in the LI-RADS lexicon, but several studies have demonstrated its importance as a predictor of MVI.27,66-68 It is hypothesized that this feature reflects peritumoral perfusion alteration caused by microscopic tumor thrombi in peritumoral portal venules, resulting in decreased OATP8 function in hepatocytes around the tumor.66
- Although the sensitivity of peritumoral hypointensity in the hepatobiliary phase of MVI is low (31.7–38.0%), its specificity is reported to be high (92.5–93.2%).27,66 A few recent studies also reported it as a significant predictor of early tumor recurrence or shorter disease-free survival after curative resection, radiofrequency ablation, or liver transplantation, when applied in combination with other imaging findings (such as arterial phase peritumoral enhancement, satellite nodule, ill-defined tumor margin) and clinical findings (such as elevated serum AFP or PIVKA-II).27,67-70
- 3. Non-smooth tumor margin
- Non-smooth tumor margin indicates that the tumor has a minute budding portion at its periphery protruding into the liver parenchyma in the hepatobiliary phase (Figs. 2, 4, 6).71 This is thought to reflect histopathologic single nodular with extranodular growth type or confluent multinodular type, which are known to have a higher risk of MVI than the single nodular type.27,72,73
- It has been reported as an independent predictor of MVI,27,34,40,74,75 progenitor subtype,76 macrotrabecular-massive subtype,10 and early recurrence after curative resection.24,27,46,71
IMAGING FINDINGS OF HEPATOBILIARY PHASE
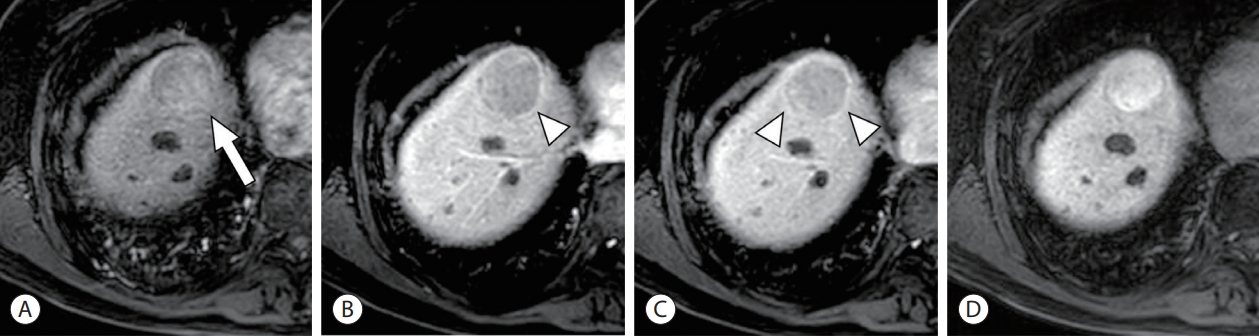
- A low apparent diffusion coefficient (ADC) is a common radiological finding observed in various malignant tumors. Theoretically, as cell density increases and the nucleus/cytoplasm ratio increases in neoplastic tissue, it results in decreased free diffusion of water molecules, which can lead to increased signal intensity in diffusion-weighted imaging (DWI) and a decrease in the ADC value (Fig. 3).77 Both the increased DWI signal and decreased ADC value suggest restricted diffusion of water molecules, but the DWI signal intensity also reflects the T2 signal. In contrast, as ADC excludes the effect of the T2 signal from DWI, it is regarded as a reliable index that more accurately depicts diffusion restriction. Contrarily, intratumoral necrosis can decrease signal intensity in DWI and increase ADC values.78
- Low ADC values have been reported to be related to poorer histological grade,79-81 presence of MVI,82-84 expression of progenitor cell markers,85,86 and proliferative signatures of HCC.87-89 A few studies have reported that a low ADC value is a significant predictor of early recurrence after curative resection of HCC.79,90
- ADC values are quantitative, but ADC values and diffusion-weighted signal intensities vary substantially depending on the imaging technique and MRI scanner; therefore, it is difficult to suggest a generalizable cutoff value that can be used clinically.91 Additionally, HCC is frequently accompanied by necrosis, and the ADC value may vary significantly depending on the location of the measurement within the lesion.
LOW APPARENT DIFFUSION COEFFICIENT ON DIFFUSION-WEIGHTED IMAGE
- LR-M is a diagnostic category of LI-RADS for malignant tumors, not definitely HCC. Tumors of LR-M include targetoid masses or non-targetoid masses with the following appearances: infiltrative appearance, marked diffusion restriction, necrosis or severe ischemia, and other features suggestive of non-HCC malignancy.13 A targetoid appearance (concentric arrangement of internal components), reflects peripheral hypercellularity, central stromal fibrosis, or ischemia, which are observed in various phases or sequences. This includes peripheral washout, delayed central enhancement (Fig. 4), targetoid diffusion restriction (Fig. 3), and targetoid appearance in the transitional or hepatobiliary phase, in addition to the rim-APHE described above. Although it is frequently reported in non-HCC malignancies, such as intrahepatic cholangiocarcinoma, combined hepatocellular cholangiocarcinoma, or metastasis, 22–36% of LR-M lesions were found to be HCC, because of the high prevalence of HCC in patients with underlying chronic liver disease.92-95
- HCCs showing LR-M features have been reported to be associated with MVI,67 poor histological differentiation.96 and stem/progenitor marker expression, including K19 and EpCAM.76,97 LR-M HCCs are likely to have a higher risk of early recurrence and poor overall survival after resection and a higher risk of tumor recurrence five years after liver transplantation.36,67,96,98 A study by Choi et al.98 demonstrated such trends in all primary liver cancers, as well as HCCs with LR-M features. Recently, Moon et al.99 reported that the presence of rim-APHE was an independent prognostic factor for postoperative survival, while the 5-year overall survival and recurrence-free survival of LR-M HCCs without rimAPHE were not different from those of LR-4/5 HCCs. However, whether rim-APHE is a more important prognostic factor than the other LR-M features needs to be validated in other studies.
LR-M CATEGORY OF LI-RADS
- Several imaging findings that indicate a poor prognosis, such as rim-APHE and non-smooth tumor margins, arterial phase peritumoral enhancement, and peritumoral hypointensity in the hepatobiliary phase, usually appear in combination. Currently, there is no consensus regarding which of these imaging findings is the most significant prognostic factor. One of the reasons for this variability may be that different studies use different definitions for imaging findings, and a more standardized definition of each imaging finding is needed to ensure consistent evaluation and validation of their prognostic significance. Moreover, the substantial interobserver variability of these imaging results has been identified as a drawback. Min et al.100 reported that there was considerable interobserver variability in each radiologic finding or combination (κ=0.38–0.47) and predicted the probability of MVI based on imaging findings (κ=0.41) evaluated on gadoxetate-enhanced MRI. For HCCs <3 cm, there was no difference in interobserver agreement for MVI according to the reviewers’ experience (κ=0.43 vs. 0.47, P>0.999), while for HCCs >3 cm, more experienced reviewers showed higher agreement than the less experienced reviewers (κ=0.65 vs. 0.21, P<0.001). Quantitative imaging analysis such as radiomics, can be utilized to reduce interobserver variability; however, the results of quantitative analysis vary significantly with imaging instruments and protocols of imaging studies.
- The majority of research has been conducted in Northeast Asia, where hepatitis B is the most prevalent cause of liver disease, and the prognosis of patients with favorable liver function who underwent hepatic resection has been investigated. In addition, most studies to date have been conducted retrospectively at a single institution with relatively small sample sizes. Therefore, it is not clear whether it can be generalized and applied to Western patients or to advanced HCCs not undergoing hepatic resection. Currently, these predictive imaging results are not incorporated into major HCC treatment guidelines because there are no well-validated imaging findings. A large-scale, multicenter study is needed in the future.
CURRENT LIMITATIONS AND THE NEED FOR FUTURE RESEARCH
- Recent studies have demonstrated the possibility that imaging features can predict not only pathological findings, such as histologic grade, presence of MVI, and pathological subtypes of HCC, but also the risk of tumor recurrence and survival. The imaging phenotype consisting of each radiological feature and/or its combination has the potential to be used for individualized treatment decisions in the future.
CONCLUSION
-
Conflicts of Interest
Hyungjin Rhee is an editorial board member of Journal of Liver Cancer, and was not involved in the review process of this article. Shin Hye Hwang declares that she has no potential conflicts of interest to disclose.
-
Ethics Statement
This review article is fully based on the articles which was already published and did not involve additional patient participants. Therefore, IRB approval is not necessary.
-
Funding Statement
This study was supported by a grant from the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2020R1C1C1003887).
-
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
-
Author Contribution
Conceptualization: HSH, HR
Writing-original draft: HSH, HR
Writing-review & editing: HSH, HR
Approval of final manuscript: HSH, HR
Article information
HCC, hepatocellular carcinoma; MVI, microvascular invasion; APHE, arterial phase hyperenhancement; VETC, vessels that encapsulate tumor cluster; CAIX, carbonic anhydrase IX; K19, keratin 19; EpCAM, epithelial cell adhesion molecule; TACE, transarterial chemoembolization; HBP, hepatobiliary phase; AFP, α-fetoprotein; ADC, apparent diffusion coefficient.
- 1. Torbenson MS, Ng IOL, Park YN, Roncalli M, Sakamoto M. Hepatocellular carcinoma. Edited by WHO Classification of Tumours Editorial Board: Digestive system tumours. 5th ed. Lyon, International Agency for Research on Cancer. 2019, pp 229−239.
- 2. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, GarciaCriado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 2022;76:681−693.ArticlePubMedPMC
- 3. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017;67:93−99.ArticlePubMedPDF
- 4. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong liver cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014;146:1691−1700.e3.ArticlePubMed
- 5. Kitao A, Zen Y, Matsui O, Gabata T, Kobayashi S, Koda W, et al. Hepatocellular carcinoma: signal intensity at gadoxetic acid-enhanced MR Imaging--correlation with molecular transporters and histopathologic features. Radiology 2010;256:817−826.PubMed
- 6. Korean Liver Cancer Association (KLCA), National Cancer Center (NCC). 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. J Liver Cancer 2022;Dec 9 . doi: 10.17998/jlc.2022.11.07. [Epub ahead of print].ArticlePDF
- 7. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM et al. diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723−750.ArticlePubMedPDF
- 8. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182−236.ArticlePubMed
- 9. Jeon Y, Kwon SM, Rhee H, Yoo JE, Chung T, Woo HG, et al. Molecular and radiopathologic spectrum between HCC and intrahepatic cholangiocarcinoma. Hepatology 2023;77:92−108.ArticlePubMedPDF
- 10. Rhee H, Cho ES, Nahm JH, Jang M, Chung YE, Baek SE, et al. Gadoxetic acid-enhanced MRI of macrotrabecular-massive hepatocellular carcinoma and its prognostic implications. J Hepatol 2021;74:109−121.ArticlePubMed
- 11. Kang HJ, Kim H, Lee DH, Hur BY, Hwang YJ, Suh KS, et al. Gadoxetate-enhanced MRI features of proliferative hepatocellular carcinoma are prognostic after surgery. Radiology 2021;300:572−582.ArticlePubMed
- 12. Rhee H, Kim H, Park YN. Clinico-radio-pathological and molecular features of hepatocellular carcinomas with keratin 19 expression. Liver Cancer 2020;9:663−681.ArticlePubMedPMCPDF
- 13. American College of Radiology. LI-RADS® CT/MRI [Internet]. Reston (VA): American College of Radiology; 2018;[cited 2022 Dec 26]. Available from: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/LI-RADS-CT-MRI-v2018
- 14. Jha RC, Mitchell DG, Weinreb JC, Santillan CS, Yeh BM, Francois R, et al. LI-RADS categorization of benign and likely benign findings in patients at risk of hepatocellular carcinoma: a pictorial atlas. AJR Am J Roentgenol 2014;203:W48−W69.ArticlePubMed
- 15. Park HJ, Jang KM, Kang TW, Song KD, Kim SH, Kim YK, et al. Identification of imaging predictors discriminating different primary liver tumours in patients with chronic liver disease on gadoxetic acid-enhanced MRI: a classification tree analysis. Eur Radiol 2016;26:3102−3111.ArticlePubMedPDF
- 16. Kadoya M, Matsui O, Takashima T, Nonomura A. Hepatocellular carcinoma: correlation of MR imaging and histopathologic findings. Radiology 1992;183:819−825.ArticlePubMed
- 17. Kutami R, Nakashima Y, Nakashima O, Shiota K, Kojiro M. Pathomorphologic study on the mechanism of fatty change in small hepatocellular carcinoma of humans. J Hepatol 2000;33:282−289.ArticlePubMed
- 18. Shibahara J, Ando S, Sakamoto Y, Kokudo N, Fukayama M. Hepatocellular carcinoma with steatohepatitic features: a clinicopathological study of Japanese patients. Histopathology 2014;64:951−962.ArticlePubMed
- 19. Qin J, Higashi T, Nakagawa S, Fujiwara N, Yamashita YI, Beppu T, et al. Steatohepatitic variant of hepatocellular carcinoma is associated with both alcoholic steatohepatitis and nonalcoholic steatohepatitis: a study of 2 cohorts with molecular insights. Am J Surg Pathol 2020;44:1406−1412.PubMedPMC
- 20. Aykutlu U, Argon A, Orman M, Ulukaya S, Zeytunlu M, Karasu Z, et al. Steatotic and steatohepatitic hepatocellular carcinomas: features in a series with predominantly viral etiology. Am J Surg Pathol 2021;45:1252−1263.PubMed
- 21. Min JH, Kim YK, Lim S, Jeong WK, Choi D, Lee WJ. Prediction of microvascular invasion of hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging: impact of intra-tumoral fat detected on chemical-shift images. Eur J Radiol 2015;84:1036−1043.ArticlePubMed
- 22. Salomao M, Remotti H, Vaughan R, Siegel AB, Lefkowitch JH, Moreira RK. The steatohepatitic variant of hepatocellular carcinoma and its association with underlying steatohepatitis. Hum Pathol 2012;43:737−746.ArticlePubMed
- 23. Siripongsakun S, Lee JK, Raman SS, Tong MJ, Sayre J, Lu DS. MRI detection of intratumoral fat in hepatocellular carcinoma: potential biomarker for a more favorable prognosis. AJR Am J Roentgenol 2012;199:1018−1025.ArticlePubMed
- 24. Chen J, Zhou J, Kuang S, Zhang Y, Xie S, He B, et al. Liver imaging reporting and data system category 5: MRI predictors of microvascular invasion and recurrence after hepatectomy for hepatocellular carcinoma. AJR Am J Roentgenol 2019;213:821−830.ArticlePubMed
- 25. Hermida M, Preel A, Assenat E, Piron L, Cassinotto C, UrsicBedoya J, et al. Small steatotic HCC: a radiological variant associated with improved outcome after ablation. Hepatol Commun 2020;5:689−700.ArticlePubMedPMCPDF
- 26. Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology 2014;272:635−654.ArticlePubMedPMC
- 27. Lee S, Kim SH, Lee JE, Sinn DH, Park CK. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol 2017;67:526−534.ArticlePubMed
- 28. An C, Kim DW, Park YN, Chung YE, Rhee H, Kim MJ. Single hepatocellular carcinoma: preoperative MR imaging to predict early recurrence after curative resection. Radiology 2015;276:433−443.ArticlePubMed
- 29. Kawamura Y, Ikeda K, Seko Y, Hosaka T, Kobayashi M, Saitoh S, et al. Heterogeneous type 4 enhancement of hepatocellular carcinoma on dynamic CT is associated with tumor recurrence after radiofrequency ablation. AJR Am J Roentgenol 2011;197:W665−W673.ArticlePubMed
- 30. Rhee H, An C, Kim HY, Yoo JE, Park YN, Kim MJ. Hepatocellular carcinoma with irregular rim-like arterial phase hyperenhancement: more aggressive pathologic features. Liver Cancer 2019;8:24−40.ArticlePubMedPMCPDF
- 31. Fang JH, Zhou HC, Zhang C, Shang LR, Zhang L, Xu J, et al. A novel vascular pattern promotes metastasis of hepatocellular carcinoma in an epithelial-mesenchymal transition-independent manner. Hepatology 2015;62:452−465.ArticlePubMed
- 32. Renne SL, Woo HY, Allegra S, Rudini N, Yano H, Donadon M, et al. Vessels Encapsulating Tumor Clusters (VETC) is a powerful predictor of aggressive hepatocellular carcinoma. Hepatology 2020;71:183−195.ArticlePubMedPDF
- 33. Kitao A, Matsui O, Zhang Y, Ogi T, Nakada S, Sato Y, et al. Dynamic CT and gadoxetic acid-enhanced MRI characteristics of P53-mutated hepatocellular carcinoma. Radiology 2023;306:e220531.ArticlePubMed
- 34. Hong SB, Choi SH, Kim SY, Shim JH, Lee SS, Byun JH, et al. MRI features for predicting microvascular invasion of hepatocellular carcinoma: a systematic review and meta-analysis. Liver Cancer 2021;10:94−106.ArticlePubMedPMCPDF
- 35. Kierans AS, Leonardou P, Hayashi P, Brubaker LM, Elazzazi M, Shaikh F, et al. MRI findings of rapidly progressive hepatocellular carcinoma. Magn Reson Imaging 2010;28:790−796.ArticlePubMed
- 36. An C, Park S, Chung YE, Kim DY, Kim SS, Kim MJ, et al. Curative resection of single primary hepatic malignancy: liver imaging reporting and data system category LR-M portends a worse prognosis. AJR Am J Roentgenol 2017;209:576−583.ArticlePubMed
- 37. Kim BK, Kim KA, An C, Yoo EJ, Park JY, Kim DY, et al. Prognostic role of magnetic resonance imaging vs. computed tomography for hepatocellular carcinoma undergoing chemoembolization. Liver Int 2015;35:1722−1730.ArticlePubMedPDF
- 38. Fowler KJ, Burgoyne A, Fraum TJ, Hosseini M, Ichikawa S, Kim S, et al. Pathologic, molecular, and prognostic radiologic features of hepatocellular carcinoma. Radiographics 2021;41:1611−1631.ArticlePubMed
- 39. Matsui O, Kobayashi S, Sanada J, Kouda W, Ryu Y, Kozaka K, et al. Hepatocelluar nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted CT) with special reference to multistep hepatocarcinogenesis. Abdom Imaging 2011;36:264−272.ArticlePubMedPMC
- 40. Renzulli M, Brocchi S, Cucchetti A, Mazzotti F, Mosconi C, Sportoletti C, et al. Can current preoperative imaging be used to detect microvascular invasion of hepatocellular carcinoma? Radiology 2016;279:432−442.ArticlePubMed
- 41. Nishie A, Yoshimitsu K, Asayama Y, Irie H, Tajima T, Hirakawa M, et al. Radiologic detectability of minute portal venous invasion in hepatocellular carcinoma. AJR Am J Roentgenol 2008;190:81−87.ArticlePubMed
- 42. Kim H, Park MS, Choi JY, Park YN, Kim MJ, Kim KS, et al. Can microvessel invasion of hepatocellular carcinoma be predicted by pre-operative MRI? Eur Radiol 2009;19:1744−1751.ArticlePubMedPDF
- 43. Yang H, Han P, Huang M, Yue X, Wu L, Li X, et al. The role of gadoxetic acid-enhanced MRI features for predicting microvascular invasion in patients with hepatocellular carcinoma. Abdom Radiol (NY) 2022;47:948−956.ArticlePubMedPDF
- 44. Wei H, Yang T, Chen J, Duan T, Jiang H, Song B. Prognostic implications of CT/MRI LI-RADS in hepatocellular carcinoma: state of the art and future directions. Liver Int 2022;42:2131−2144.PubMed
- 45. Wei H, Jiang H, Liu X, Qin Y, Zheng T, Liu S, et al. Can LI-RADS imaging features at gadoxetic acid-enhanced MRI predict aggressive features on pathology of single hepatocellular carcinoma? Eur J Radiol 2020;132:109312. ArticlePubMed
- 46. Zhang L, Kuang S, Chen J, Zhang Y, Zhao B, Peng H, et al. The role of preoperative dynamic contrast-enhanced 3.0-T MR imaging in predicting early recurrence in patients with early-stage hepatocellular carcinomas after curative resection. Front Oncol 2019;9:1336. ArticlePubMedPMC
- 47. Kitao A, Zen Y, Matsui O, Gabata T, Nakanuma Y. Hepatocarcinogenesis: multistep changes of drainage vessels at CT during arterial portography and hepatic arteriography--radiologic-pathologic correlation. Radiology 2009;252:605−614.ArticlePubMed
- 48. Rhee H, Chung T, Yoo JE, Nahm JH, Woo HY, Choi GH, et al. Gross type of hepatocellular carcinoma reflects the tumor hypoxia, fibrosis, and stemness-related marker expression. Hepatol Int 2020;14:239−248.ArticlePubMedPDF
- 49. Ishizaki M, Ashida K, Higashi T, Nakatsukasa H, Kaneyoshi T, Fujiwara K, et al. The formation of capsule and septum in human hepatocellular carcinoma. Virchows Arch 2001;438:574−580.ArticlePubMedPDF
- 50. Iguchi T, Aishima S, Sanefuji K, Fujita N, Sugimachi K, Gion T, et al. Both fibrous capsule formation and extracapsular penetration are powerful predictors of poor survival in human hepatocellular carcinoma: a histological assessment of 365 patients in Japan. Ann Surg Oncol 2009;16:2539−2546.ArticlePubMedPDF
- 51. Ishigami K, Yoshimitsu K, Nishihara Y, Irie H, Asayama Y, Tajima T, et al. Hepatocellular carcinoma with a pseudocapsule on gadolinium-enhanced MR images: correlation with histopathologic findings. Radiology 2009;250:435−443.ArticlePubMed
- 52. An C, Rhee H, Han K, Choi JY, Park YN, Park MS, et al. Added value of smooth hypointense rim in the hepatobiliary phase of gadoxetic acid-enhanced MRI in identifying tumour capsule and diagnosing hepatocellular carcinoma. Eur Radiol 2017;27:2610−2618.ArticlePubMedPDF
- 53. Wakasa K, Sakurai M, Kuroda C, Marukawa T, Monden M, Okamura J, et al. Effect of transcatheter arterial embolization on the boundary architecture of hepatocellular carcinoma. Cancer 1990;65:913−919.ArticlePubMed
- 54. Wu TH, Yu MC, Chen TC, Lee CF, Chan KM, Wu TJ, et al. Encapsulation is a significant prognostic factor for better outcome in large hepatocellular carcinoma. J Surg Oncol 2012;105:85−90.ArticlePubMedPDF
- 55. Lai EC, Ng IO, Ng MM, Lok AS, Tam PC, Fan ST, et al. Longterm results of resection for large hepatocellular carcinoma: a multivariate analysis of clinicopathological features. Hepatology 1990;11:815−818.ArticlePubMed
- 56. Witjes CD, Willemssen FE, Verheij J, van der Veer SJ, Hansen BE, Verhoef C, et al. Histological differentiation grade and microvascular invasion of hepatocellular carcinoma predicted by dynamic contrast-enhanced MRI. J Magn Reson Imaging 2012;36:641−647.ArticlePubMed
- 57. Bartolozzi C, Battaglia V, Bargellini I, Bozzi E, Campani D, Pollina LE, et al. Contrast-enhanced magnetic resonance imaging of 102 nodules in cirrhosis: correlation with histological findings on explanted livers. Abdom Imaging 2013;38:290−296.ArticlePubMedPDF
- 58. Kim JY, Kim MJ, Kim KA, Jeong HT, Park YN. Hyperintense HCC on hepatobiliary phase images of gadoxetic acid-enhanced MRI: correlation with clinical and pathological features. Eur J Radiol 2012;81:3877−3882.ArticlePubMed
- 59. Yamashita T, Kitao A, Matsui O, Hayashi T, Nio K, Kondo M, et al. Gd-EOB-DTPA-enhanced magnetic resonance imaging and alpha-fetoprotein predict prognosis of early-stage hepatocellular carcinoma. Hepatology 2014;60:1674−1685.ArticlePubMedPMC
- 60. Kitao A, Matsui O, Yoneda N, Kozaka K, Kobayashi S, Koda W, et al. Gadoxetic acid-enhanced MR imaging for hepatocellular carcinoma: molecular and genetic background. Eur Radiol 2020;30:3438−3447.ArticlePubMedPDF
- 61. Ba-Ssalamah A, Antunes C, Feier D, Bastati N, Hodge JC, Stift J, et al. Morphologic and molecular features of hepatocellular adenoma with gadoxetic acid-enhanced MR imaging. Radiology 2015;277:104−113.ArticlePubMed
- 62. Grazioli L, Bondioni MP, Haradome H, Motosugi U, Tinti R, Frittoli B, et al. Hepatocellular adenoma and focal nodular hyperplasia: value of gadoxetic acid-enhanced MR imaging in differential diagnosis. Radiology 2012;262:520−529.ArticlePubMed
- 63. Kitao A, Matsui O, Yoneda N, Kozaka K, Kobayashi S, Koda W, et al. Hypervascular hepatocellular carcinoma: correlation between biologic features and signal intensity on gadoxetic acid-enhanced MR images. Radiology 2012;265:780−789.ArticlePubMed
- 64. Yoneda N, Matsui O, Kitao A, Kita R, Kozaka K, Koda W, et al. Hypervascular hepatocellular carcinomas showing hyperintensity on hepatobiliary phase of gadoxetic acid-enhanced magnetic resonance imaging: a possible subtype with mature hepatocyte nature. Jpn J Radiol 2013;31:480−490.ArticlePubMedPDF
- 65. Choi JW, Lee JM, Kim SJ, Yoon JH, Baek JH, Han JK, et al. Hepatocellular carcinoma: imaging patterns on gadoxetic acid-enhanced MR images and their value as an imaging biomarker. Radiology 2013;267:776−786.ArticlePubMed
- 66. Kim KA, Kim MJ, Jeon HM, Kim KS, Choi JS, Ahn SH, et al. Prediction of microvascular invasion of hepatocellular carcinoma: usefulness of peritumoral hypointensity seen on gadoxetate disodiumenhanced hepatobiliary phase images. J Magn Reson Imaging 2012;35:629−634.ArticlePubMed
- 67. Lee S, Kim KW, Jeong WK, Jeong SY, Hwang JA, Choi JS, et al. Liver imaging reporting and data system category on magnetic resonance imaging predicts recurrence of hepatocellular carcinoma after liver transplantation within the milan criteria: a multicenter study. Ann Surg Oncol 2021;28:6782−6789.ArticlePubMedPDF
- 68. Wei H, Jiang H, Zheng T, Zhang Z, Yang C, Ye Z, et al. LI-RADS category 5 hepatocellular carcinoma: preoperative gadoxetic acidenhanced MRI for early recurrence risk stratification after curative resection. Eur Radiol 2021;31:2289−2302.ArticlePubMedPMCPDF
- 69. Kang TW, Rhim H, Lee J, Song KD, Lee MW, Kim YS, et al. Magnetic resonance imaging with gadoxetic acid for local tumour progression after radiofrequency ablation in patients with hepatocellular carcinoma. Eur Radiol 2016;26:3437−3446.ArticlePubMedPDF
- 70. Kim AY, Sinn DH, Jeong WK, Kim YK, Kang TW, Ha SY, et al. Hepatobiliary MRI as novel selection criteria in liver transplantation for hepatocellular carcinoma. J Hepatol 2018;68:1144−1152.ArticlePubMed
- 71. Ariizumi S, Kitagawa K, Kotera Y, Takahashi Y, Katagiri S, Kuwatsuru R, et al. A non-smooth tumor margin in the hepatobiliary phase of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging predicts microscopic portal vein invasion, intrahepatic metastasis, and early recurrence after hepatectomy in patients with hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2011;18:575−585.ArticlePubMedPDF
- 72. Nakashima Y, Nakashima O, Tanaka M, Okuda K, Nakashima M, Kojiro M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res 2003;26:142−147.ArticlePubMed
- 73. Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol 2008;15:1375−1382.ArticlePubMedPDF
- 74. Chou CT, Chen RC, Lin WC, Ko CJ, Chen CB, Chen YL. Prediction of microvascular invasion of hepatocellular carcinoma: preoperative CT and histopathologic correlation. AJR Am J Roentgenol 2014;203:W253−W259.ArticlePubMed
- 75. Jiang H, Wei J, Fu F, Wei H, Qin Y, Duan T, et al. Predicting microvascular invasion in hepatocellular carcinoma: a dualinstitution study on gadoxetate disodium-enhanced MRI. Liver Int 2022;42:1158−1172.PubMedPMC
- 76. Chen J, Wu Z, Xia C, Jiang H, Liu X, Duan T, et al. Noninvasive prediction of HCC with progenitor phenotype based on gadoxetic acid-enhanced MRI. Eur Radiol 2020;30:1232−1242.ArticlePubMedPDF
- 77. Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology 2010;254:47−66.ArticlePubMed
- 78. Deng J, Rhee TK, Sato KT, Salem R, Haines K, Paunesku T, et al. In vivo diffusion-weighted imaging of liver tumor necrosis in the VX2 rabbit model at 1.5 Tesla. Invest Radiol 2006;41:410−414.ArticlePubMed
- 79. Nakanishi M, Chuma M, Hige S, Omatsu T, Yokoo H, Nakanishi K, et al. Relationship between diffusion-weighted magnetic resonance imaging and histological tumor grading of hepatocellular carcinoma. Ann Surg Oncol 2012;19:1302−1309.ArticlePubMedPDF
- 80. Nishie A, Tajima T, Asayama Y, Ishigami K, Kakihara D, Nakayama T, et al. Diagnostic performance of apparent diffusion coefficient for predicting histological grade of hepatocellular carcinoma. Eur J Radiol 2011;80:e29−e33.ArticlePubMed
- 81. Heo SH, Jeong YY, Shin SS, Kim JW, Lim HS, Lee JH, et al. Apparent diffusion coefficient value of diffusion-weighted imaging for hepatocellular carcinoma: correlation with the histologic differentiation and the expression of vascular endothelial growth factor. Korean J Radiol 2010;11:295−303.ArticlePubMedPMC
- 82. Suh YJ, Kim MJ, Choi JY, Park MS, Kim KW. Preoperative prediction of the microvascular invasion of hepatocellular carcinoma with diffusion-weighted imaging. Liver Transpl 2012;18:1171−1178.ArticlePubMed
- 83. Surov A, Pech M, Omari J, Fischbach F, Damm R, Fischbach K, et al. Diffusion-weighted imaging reflects tumor grading and microvascular invasion in hepatocellular carcinoma. Liver Cancer 2021;10:10−24.ArticlePubMedPMCPDF
- 84. Xu P, Zeng M, Liu K, Shan Y, Xu C, Lin J. Microvascular invasion in small hepatocellular carcinoma: is it predictable with preoperative diffusion-weighted imaging? J Gastroenterol Hepatol 2014;29:330−336.ArticlePubMedPDF
- 85. Jeong HT, Kim MJ, Kim YE, Park YN, Choi GH, Choi JS. MRI features of hepatocellular carcinoma expressing progenitor cell markers. Liver Int 2012;32:430−440.ArticlePubMed
- 86. Choi SY, Kim SH, Park CK, Min JH, Lee JE, Choi YH, et al. Imaging features of gadoxetic acid-enhanced and diffusion-weighted MR imaging for identifying cytokeratin 19-positive hepatocellular carcinoma: a retrospective observational study. Radiology 2018;286:897−908.ArticlePubMed
- 87. Chen J, Xia C, Duan T, Cao L, Jiang H, Liu X, et al. Macrotrabecular-massive hepatocellular carcinoma: imaging identification and prediction based on gadoxetic acid-enhanced magnetic resonance imaging. Eur Radiol 2021;31:7696−7704.ArticlePubMedPDF
- 88. Hu XX, Yang ZX, Liang HY, Ding Y, Grimm R, Fu CX, et al. Wholetumor MRI histogram analyses of hepatocellular carcinoma: correlations with Ki-67 labeling index. J Magn Reson Imaging 2017;46:383−392.ArticlePubMedPDF
- 89. Huang Z, Xu X, Meng X, Hou Z, Liu F, Hua Q, et al. Correlations between ADC values and molecular markers of Ki-67 and HIF-1α in hepatocellular carcinoma. Eur J Radiol 2015;84:2464−2469.ArticlePubMed
- 90. Lee S, Kim SH, Hwang JA, Lee JE, Ha SY. Pre-operative ADC predicts early recurrence of HCC after curative resection. Eur Radiol 2019;29:1003−1012.ArticlePubMedPDF
- 91. Sasaki M, Yamada K, Watanabe Y, Matsui M, Ida M, Fujiwara S, et al. Variability in absolute apparent diffusion coefficient values across different platforms may be substantial: a multivendor, multi-institutional comparison study. Radiology 2008;249:624−630.ArticlePubMed
- 92. van der Pol CB, Lim CS, Sirlin CB, McGrath TA, Salameh JP, Bashir MR, et al. Accuracy of the liver imaging reporting and data system in computed tomography and magnetic resonance image analysis of hepatocellular carcinoma or overall malignancy-a systematic review. Gastroenterology 2019;156:976−986.ArticlePubMed
- 93. Lee S, Kim SS, Roh YH, Choi JY, Park MS, Kim MJ. Diagnostic performance of CT/MRI liver imaging reporting and data system v2017 for hepatocellular carcinoma: a systematic review and meta-analysis. Liver Int 2020;40:1488−1497.ArticlePubMedPDF
- 94. Kim DH, Choi SH, Park SH, Kim KW, Byun JH, Kim SY, et al. Liver imaging reporting and data system category M: a systematic review and meta-analysis. Liver Int 2020;40:1477−1487.ArticlePubMedPDF
- 95. Lee S, Kim YY, Shin J, Hwang SH, Roh YH, Chung YE, et al. CT and MRI liver imaging reporting and data system version 2018 for hepatocellular carcinoma: a systematic review with meta-analysis. J Am Coll Radiol 2020;17:1199−1206.ArticlePubMed
- 96. Shin J, Lee S, Kim SS, Chung YE, Choi JY, Park MS, et al. Characteristics and early recurrence of hepatocellular carcinomas categorized as LR-M: comparison with those categorized as LR-4 or 5. J Magn Reson Imaging 2021;54:1446−1454.PubMed
- 97. Hu XX, Wang WT, Yang L, Yang ZX, Liang HY, Ding Y, et al. MR features based on LI-RADS identify cytokeratin 19 status of hepatocellular carcinomas. Eur J Radiol 2019;113:7−14.ArticlePubMed
- 98. Choi SH, Lee SS, Park SH, Kim KM, Yu E, Park Y, et al. LI-RADS classification and prognosis of primary liver cancers at gadoxetic acid-enhanced MRI. Radiology 2019;290:388−397.ArticlePubMed
- 99. Moon JY, Min JH, Kim YK, Cha D, Hwang JA, Ko SE, et al. Prognosis after curative resection of single hepatocellular carcinoma with a focus on LI-RADS targetoid appearance on preoperative gadoxetic acid-enhanced MRI. Korean J Radiol 2021;2211:1786−1796.ArticlePDF
- 100. Min JH, Lee MW, Park HS, Lee DH, Park HJ, Lim S, et al. Interobserver variability and diagnostic performance of gadoxetic acidenhanced MRI for predicting microvascular invasion in hepatocellular carcinoma. Radiology 2020;297:573−581.ArticlePubMed
References
Figure & Data
References
Citations

- Radiomics and machine learning based on preoperative MRI for predicting extrahepatic metastasis in hepatocellular carcinoma patients treated with transarterial chemoembolization
Gang Peng, Xiaojing Cao, Xiaoyu Huang, Xiang Zhou
European Journal of Radiology Open.2024; 12: 100551. CrossRef - Imaging prognostication and tumor biology in hepatocellular carcinoma
Diana Kadi, Marilyn F. Yamamoto, Emily C. Lerner, Hanyu Jiang, Kathryn J. Fowler, Mustafa R. Bashir
Journal of Liver Cancer.2023; 23(2): 284. CrossRef
 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation
- Download Citation
- Close
- Related articles
-
- Multidisciplinary approach for hepatocellular carcinoma patients: current evidence and future perspectives
- Changing etiology and epidemiology of hepatocellular carcinoma: Asia and worldwide
- Radiofrequency for hepatocellular carcinoma larger than 3 cm: potential for applications in daily practice
- Intermediate-stage hepatocellular carcinoma: refining substaging or shifting paradigm?
- Liver resection in selective hepatocellular carcinoma with Vp3 or Vp4 portal vein tumor thrombosis improves prognosis

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION


 Follow JLC on Twitter
Follow JLC on Twitter