Articles
- Page Path
- HOME > J Liver Cancer > Volume 24(1); 2024 > Article
-
Original Article
Liver resection in selective hepatocellular carcinoma with Vp3 or Vp4 portal vein tumor thrombosis improves prognosis -
Manuel Lim1
 , Jongman Kim2
, Jongman Kim2 , Jinsoo Rhu2
, Jinsoo Rhu2 , Gyu-Seong Choi2
, Gyu-Seong Choi2 , Jae-Won Joh2
, Jae-Won Joh2
-
Journal of Liver Cancer 2024;24(1):102-112.
DOI: https://doi.org/10.17998/jlc.2024.01.31
Published online: February 14, 2024
1Department of Surgery, Myoungji Hospital, Hanyang University College of Medicine, Goyang, Korea
2Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- Corresponding author: Jongman Kim, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea E-mail: yjongman21@gmail.com
© 2024 The Korean Liver Cancer Association.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 406 Views
- 38 Downloads
Abstract
-
Background/Aim
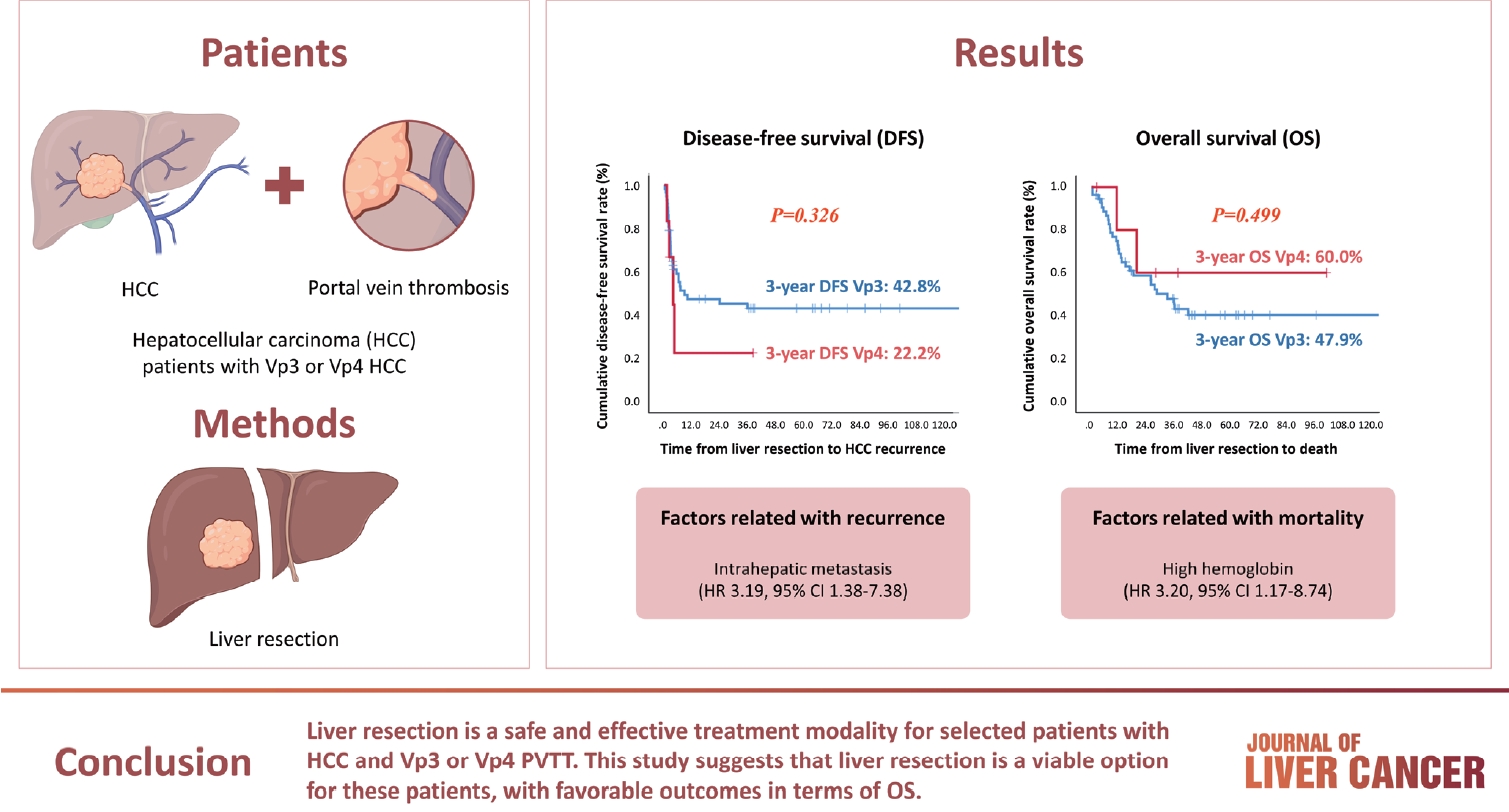
- Hepatocellular carcinoma (HCC) tumor thrombi located in the first branch of the portal vein (Vp3) or in the main portal trunk (Vp4) are associated with poor prognosis. This study aimed to investigate the clinicopathological characteristics and risk factors for HCC recurrence and mortality following liver resection (LR) in patients with Vp3 or Vp4 HCC.
-
Methods
- The study included 64 patients who underwent LR for HCC with Vp3 or Vp4 portal vein tumor thrombosis (PVTT).
-
Results
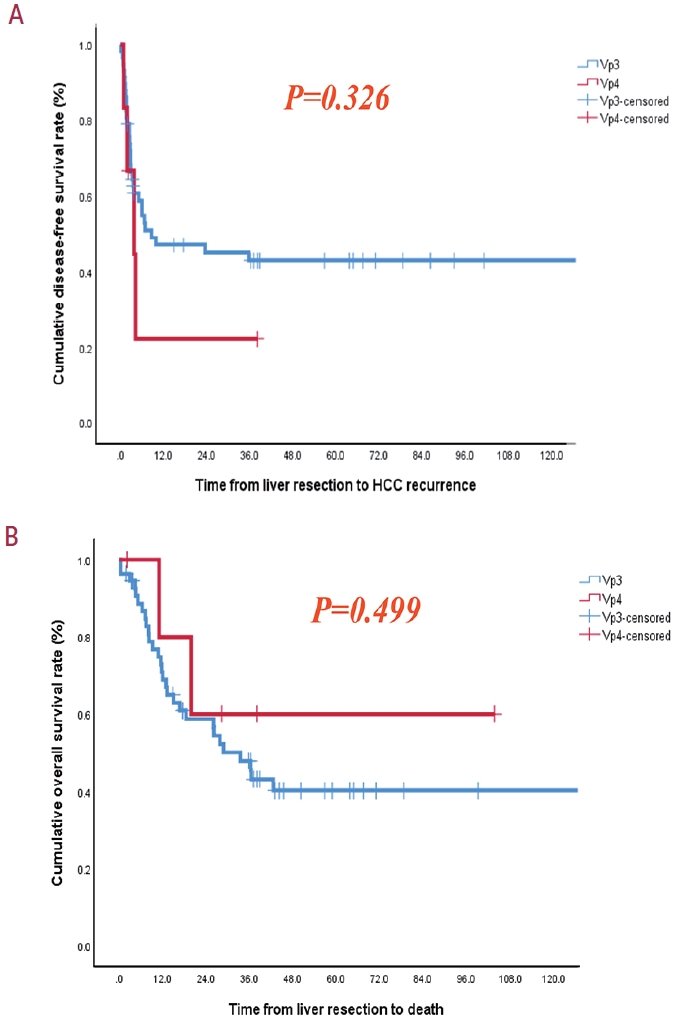
- Fifty-eight patients (90.6%) had Vp3 PVTT, whereas the remaining six patients exhibited Vp4 PVTT. The median tumor size measured 8 cm, with approximately 36% of patients presented with multiple tumors. Fifty-four patients (84.4%) underwent open LR, whereas 10 patients underwent laparoscopic LR. In the Vp4 cases, combined LR and tumor thrombectomy were performed. The 3-year cumulative disease-free survival rate was 42.8% for the Vp3 group and 22.2% for the Vp4 group. The overall survival (OS) rate at 3 years was 47.9% for the Vp3 group and 60.0% for the Vp4 group. Intrahepatic metastasis has been identified as an important contributor to HCC recurrence. High hemoglobin levels are associated with high mortality.
-
Conclusion
- LR is a safe and effective treatment modality for selected patients with Vp3 or Vp4 HCC PVTT. This suggests that LR is a viable option for these patients, with favorable outcomes in terms of OS.
- Portal vein tumor thrombosis (PVTT) is a significant complication encountered in a substantial proportion of patients with hepatocellular carcinoma (HCC). PVTT occurs when HCC invades and obstructs the portal vein and is associated with poor prognosis.1,2 This is attributed to several factors, including an increased risk of tumor spread, elevated portal pressure, reduced portal blood flow, and the potential development of complications such as variceal bleeding, ascites, and liver failure.3
- The therapeutic options for HCC PVTT patients are limited. Sorafenib can be used to prolong the overall survival (OS) and time-to-progression.4,5 Recently, a combination of atezolizumab and bevacizumab has shown promise in clinical trials and has the potential to improve outcomes in these patients.6 Individualized treatment plans and multidisciplinary approaches are crucial for managing HCC with PVTT.
- Liver resection (LR) is recognized as a potential treatment option for specific patients with HCC PVTT, especially when the tumor thrombus is situated in the first branch of the portal vein (Vp3) or the main portal trunk (Vp4).7,8 Although LR offers the potential for complete tumor removal, it remains a technically challenging procedure with a high risk of HCC recurrence and post-hepatectomy liver failure in cases of Vp3 or Vp4 PVTT. Given the challenging nature of HCC with Vp3 or Vp4 PVTT, identifying prognostic factors associated with improved outcomes has been a focal point. Appropriate patient selection, perioperative outcomes, and long-term prognostic analysis for HCC with tumor thrombus extending to the main portal trunk have not yet been evaluated.9,10 Understanding these factors can improve patient selection and the development of more effective treatment strategies.
- This study aimed to investigate the clinicopathological characteristics and risk factors associated with HCC recurrence and mortality in 64 patients who underwent LR for HCC with PVTT (Vp3 or Vp4).
INTRODUCTION
- Patients
- Between September 2002 and July 2021, 64 patients underwent LR at Samsung Medical Center. The diagnosis of HCC with PVTT (Vp3 or Vp4) was confirmed through post-operative histological examination. Patients aged ≤18 years and those with pathologically proven mixed HCC and cholangiocarcinoma, segmental portal vein thrombosis, or those lost to follow-up after LR were excluded. Demographic, preoperative laboratory, and pathological data of all patients were collected from electronic medical records and retrospectively reviewed. The function was evaluated using the Child-Pugh classification system. Certain patients with Vp3 or Vp4 underwent surgical LR after combined treatment with transarterial chemoembolization (TACE) and external beam radiation therapy (EBRT). These patients were defined as scheduled hepatectomy.
- This study received approval from the Samsung Medical Center Ethics Committee (SMC-2023-11-038) and adhered to the principles of the Declaration of Helsinki. The Institutional Review Board waived the need for patient consent due to the retrospective nature of this observational study and the use of data from patient medical records.
- Surgery and pathology
- The surgical procedures employed have been described in previous reports.7,8 For HCC with PVTT extending to the first, major, or opposite portal veins, either right or left hemihepatectomy is necessary. Prior to mobilization and liver transection, ligation of the hepatic artery and portal vein or removal of the PVTT was performed to prevent tumor scattering. PVTT localization in the first branch allows bifurcation without exposing the tumor thrombus. In cases where PVTT extends to the main or opposite side branch, interrupting portal venous flow, incision of the portal venous wall was carried out, the PVTT was removed, and the stump was closed with a continuous suture after flushing with heparin-mixed saline, ensuring that no portal venous velocity remained.
- Post-operative histological assessment of patients with HCC included tumor diameter, capsular formation, portal vein invasion, bile duct invasion, serosal involvement, intrahepatic metastasis, multicentric HCC occurrence, cirrhosis, and resection margins. The Liver Cancer Study Group of Japan classifies tumor invasion into two groups, Vp3 or Vp4,10 acknowledging potential therapeutic and prognostic differences between the groups. The histological grade of HCC was assessed according to the Edmonson-Steiner grading system, categorizing HCC as welldifferentiated (grade I), moderately differentiated (grade II), or poorly differentiated (grades III and IV).11
- Surveillance after surgical resection
- The surveillance protocol post-surgery at our center has been previously described.12,13 Abdominal computed tomography (CT) was conducted every 3 months or when recurrence was suspected. Magnetic resonance imaging and/or positron emission tomography scans were used if CT could not definitively detect recurrence. Recurrent HCCs were managed with re-resection, radiofrequency ablation, TACE, EBRT, or sorafenib, depending on liver function reserve and recurrence pattern. The follow-up time was defined as the period from surgery to the final follow-up (December 31, 2022), or death. None of the patients received adjuvant therapy following curative LR.
- Statistical analysis
- SPSS statistical software (ver. 25.0; SPSS Inc., Chicago, IL, USA) was employed to analyze data on risk factors for HCC recurrence or death in patients with Vp3 or Vp4. Continuous variables are presented as medians and ranges, whereas categorical variables were compared using Fisher’s exact test. Disease-free survival (DFS) and OS rates were calculated using the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariate Cox-regression analyses were performed to identify risk factors. A final model was created with variables having P<0.10, and a significance level of P<0.05.
METHODS
- Baseline characteristics
- Patients presenting with Vp3 PVTT (n=58) accounted for 90.6% of the cases, whereas the remaining 9.4% experienced Vp4 PVTT (n=6). The sex distribution was skewed towards male patients (n=51), constituting 79.7% of the cohort, with a median age at the time of LR being 53 years (range, 28-76). The etiological factors for HCC vary, with hepatitis B virus (HBV) infection being the most prevalent (56.3%), followed by non-B non-C causes (39.1%). One-quarter of the patients (25%) had previously undergone TACE-based locoregional therapy (Table 1).
- Perioperative and pathologic characteristics
- Classification of patients based on the American Society of Anesthesiologists (ASA) criteria revealed that 12 patients (18.8%) were ASA class I, 43 patients (67.2%) were ASA class II, and nine patients (14.1%) were ASA class III. Most patients (n=54; 84.4%) underwent open LR, whereas 10 underwent laparoscopic LR. In cases involving Vp4 PVTT, the thrombectomy was performed without additional resection. The median operation time was 263 minutes (range, 136-669), and the median blood loss during LR was 475 mL (range, 30-13,000). Three patients received intra- or post-operative transfusion of red blood cells. The median duration of hospitalization stay after LR was 10 days (range, 5-35).
- Postoperative complications were observed in eight patients, including hepatic failure (n=2), portal vein thrombosis (n=1), wound complications (n=2), pneumonia (n=2), and ascites (n=1) (Table 2). Tumor characteristics encompassed a median tumor size measuring 8.0 cm (range, 1.5-27.0), with 35.9% of patients presenting with multifocal tumors. Some patients exhibited concurrent serosal invasion (15.6%) or bile duct tumor thrombosis (18.8%). Cirrhosis of the background liver was identified in 42.2% of patients (Table 3).
- Survival and risk factors for HCC recurrence and death
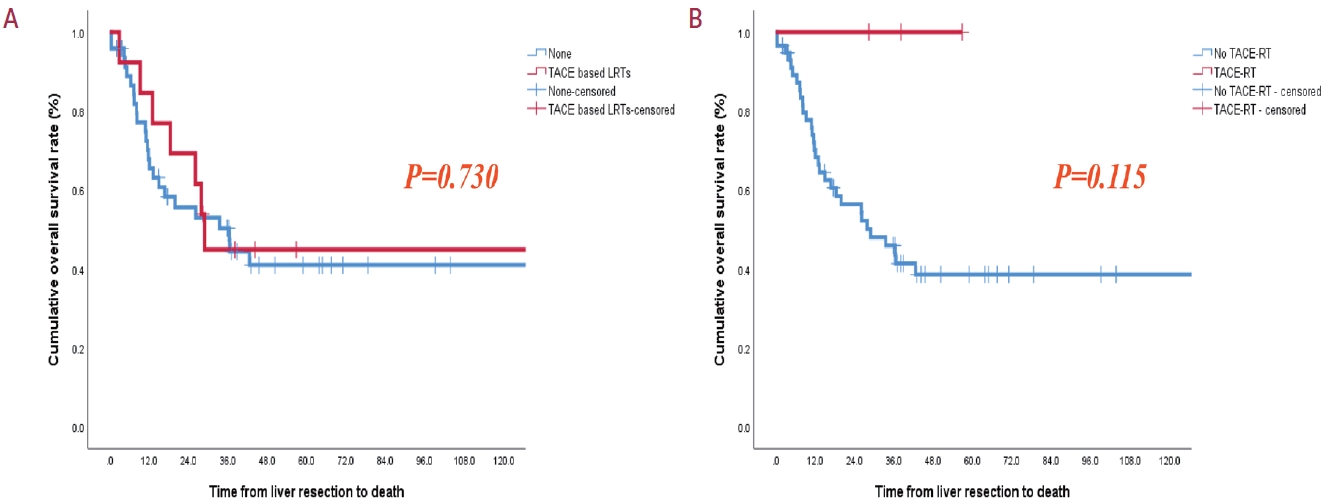
- The 3-year cumulative DFS rate was 42.8% for patients with Vp3 PVTT and 22.2% for those with Vp4 PVTT. Similarly, the 3-year OS rates were 47.9% and 60.0% for patients with Vp3 and Vp4, respectively (Fig. 1). The initial site of recurrence was within the intrahepatic region, with no patients developing an extrahepatic recurrence initially. Importantly, our analysis did not reveal any statistically significant differences in DFS or OS between the Vp3 and Vp4 groups. Intrahepatic metastasis and high hemoglobin levels were identified as important factors in the multivariate analyses of HCC recurrence and death, respectively (Tables 4 and 5). The 3-year cumulative OS rate was 44.9% for patients who underwent preoperative TACE-based locoregional therapies (LRTs) and 50.3% for those without preoperative TACE-based LRTs. Interestingly, all patients who underwent the combination treatment with TACE and EBRT with the intention of undergoing hepatectomy survived until the final visit. However, the 3-year OS rate was 45.9% in patients who did not receive combination treatment with TACE and EBRT. Scheduled hepatectomy patients displayed favorable survival outcomes following LR despite the limited number of cases and brief follow-up duration (Fig. 2).
RESULTS
- Our study yielded promising results in terms of DFS and OS following curative LR for patients with HCC PVTT at the Vp3 or Vp4 stages. LR plays a crucial role in mechanically removing PVTT obstruction and halting further progression, effectively expanding the therapeutic time window for subsequent treatments. This approach facilitates the integration of multidisciplinary treatments, contributing to extended survival in patients with HCC presenting with advanced PVTT.
- Patients with PVTT represent a diverse population with varied prognoses influenced by factors such as PVTT extent, portal hypertension severity, liver function, clinical characteristics, and treatment responses.14 Surgical intervention is warranted only in specific cases due to the potential for multiple intrahepatic metastases and compromised hepatic reserves in these patients. Consequently, non-surgical treatments such as TACE, EBRT, and systemic chemotherapy have been traditionally employed for managing patients with HCC PVTT.15-17 Unfortunately, the outcomes of these palliative interventions are often disappointing, leading to limited survival.
- Notably, a phase III randomized controlled trial demonstrated that the median survival time in patients with advanced HCC treated with sorafenib was only 6.5 months.4 Conversely, advancements in surgical techniques and perioperative management have significantly improved long-term survival outcomes in specific cases of patients with HCC PVTT who undergo aggressive surgical intervention.7,8 A Japanese multicenter study found no statistically significant difference in OS between patients with HCC PVTT Vp3 and Vp4, with respective median survival times of 24.7 and 18.1 months (P=0.1512).18 These varying survival outcomes can be attributed to differences in patient selection, surgical techniques, and the use of multidisciplinary treatments. Our study results surpassed those of the Japanese multicenter study, primarily because of our highly selective approach in treating HCC PVTT cases with preserved liver function.
- LR combined with tumor thrombectomy is considered the most effective procedure for preventing acute portal hypertension and hepatic failure in patients with PVTT.10,19 A tumor thrombus located in Vp3 can be completely eliminated by performing a major LR coupled with the division of the ipsilateral portal branch near the bifurcation. For addressing Vp4 tumor thrombus, thrombectomy or en bloc resection of the main portal trunk may be necessary. The latter approach involves the resection and reconstruction of the main portal vein in addition to LR. Noting the limited cases of resectable Vp4 cases is important, our study identified only six such instances. Surgical intervention is generally not recommended for Vp4 cases due to the higher risk and poorer prognosis associated with this condition compared with Vp3 cases. Surgical procedures for Vp4 cases are often considered non-curative resections.9,20 In this study, we did not perform en bloc resection of the main portal trunk. Instead, we opted for tumor thrombectomy exclusively for Vp4 cases. Although thrombectomy might be perceived as a potentially non-curative resection due to the risk of exposing tumor cells in the surgical field, it has demonstrated comparable outcomes to those achieved with en bloc resection in terms of morbidity, mortality, and survival. This is especially evident in cases of PVTT extending beyond the bifurcation or into other liver segments.19
- A notable concern following thrombectomy is the potential for peritoneal recurrence due to tumor dissemination within the surgical field. The incidence of peritoneal recurrence has been reported to be as high as 45.5% in patients with ruptured HCC,21 but is notably lower (3.4%) following thrombectomy for bile duct tumor thrombus.22 Fortunately, our study did not observe peritoneal seeding as the initial site of recurrence following LR. Despite encountering severe post-operative complications, such as liver failure (two cases) and portal vein thrombosis (one case), LR ultimately provided the potential for long-term survival in selected patients.
- The prognosis of HCC is often grim due to intrahepatic metastasis and recurrence, strongly associated with PVTT.23,24 Interestingly, in our study, the degree of PVTT did not emerge as the most critical prognostic factor; instead, intrahepatic metastases and high hemoglobin levels played pivotal roles. Although only a small proportion of patients with HCC PVTT have achieved favorable outcomes,7,8 our analysis focused on identifying the key prognostic factors associated with these improved results.
- The presence of PVTT in HCC indicates that cancer cells have already spread through the portal vein system, leading to the formation of satellite nodules, multiple intrahepatic metastases, and distant hematogenous metastases. Intrahepatic metastatic recurrence is almost inevitable and life-threatening in patients with Vp3 or Vp4 PVTT.10,19,20 Managing these recurrence patterns can significantly impact postoperative survival, as multiple intrahepatic recurrences can rapidly spread to the remaining liver along with the tumor thrombus.20 Immediate multiple hepatic recurrences following LR have been associated with poorer OS in patients with HCC PVTT.7,8,10,19,20,25
- Hypoxia has been identified as a contributing factor to various types of cancer, and anemia has been suggested to be associated with tumor hypoxia.26 Anemia is a common complication in HCC patients, and previous evidence has indicated its correlated with poor clinical prognosis in HCC.27 Elevated hemoglobin levels may be a compensatory response to the presence of massive tumors that have outgrown their vascular supply, leading to local ischemia and hypoxia.28 The association between high hemoglobin levels and poor survival observed in our study suggests that this phenomenon may serve as a prognostic indicator. Although the exact mechanism underlying this relationship remains unclear, advanced PVTT may contribute to increased erythropoiesis, and a high hemoglobin level in HCC with PVTT could reflect the aggressive biology of the tumor. Further research is needed to fully understand the underlying mechanisms and validate hemoglobin levels as a prognostic factor in this context.
- The frequent involvement of the portal vein (PV) in HCC is primarily attributed to the intrahepatic PV branches serving as the outflow pathways for growing HCC lesions, predominantly supplied by hepatic arterial blood flow. This dynamic leads to the transport spread of tumor cells into the PV system.14,29 Previous studies have emphasized the potential benefits of preoperative TACE in patients with HCC PVTT.30,31 However, the effectiveness of preoperative TACE may be limited in cases where the tumor thrombus grows rapidly, leading to portal hypertension and acute liver failure.25 A combination of nonsurgical treatments and LR, either with curative or palliative intent, has been employed to improve OS. Systemic chemotherapy, EBRT, or a combination of both are preferred.32,33 A recent study concluded that combined TACE and radiation therapy (RT), rather than systemic treatment alone, may be considered as a first-line treatment option for patients with HCC and macrovascular invasion.34 A previous randomized controlled trial and systematic review reported that neoadjuvant RT provided significantly better post-operative survival outcomes than surgery alone in patients with resectable HCC and PVTT. Our study also demonstrated that even in patients with resectable Vp3 or Vp4 HCC PVTT, scheduled hepatectomy improved OS when combined with TACE and EBRT as neoadjuvant therapy.32,35 Our study revealed that preoperative TACE and EBRT might effectively prevent tumor spread and influence the decision to perform surgical LR. Although the difference between the two groups was insignificant and small, it indicates the possibility of a good prognosis (Fig. 2). In cases where a downstaging effect was observed following these treatments, LR was considered for patients with resectable HCC lesions and preserved hepatic function. This finding suggests that significant downstaging, resulting from prior treatment, can serve as a patient selection criterion for LR. Conversely, patients who experience tumor progression despite previous locoregional treatments may not be suitable candidates for LR.
- This study has several limitations. First, being a retrospective single-center cohort study, comes with inherent limitations such as potential selection biases, an exclusive focus on hepatectomy patients, and the inability to establish causal relationships. Optimal comparison would involve the sorafenib treatment and LR groups, but identifying a suitable sorafenib treatment group as the control group proved challenging. Therefore, we exclusively included hepatectomy patients with HCC PVTT Vp3 or Vp4. Second, the data from our surgical cohort may be insufficient to definitively determine the optimal treatment strategy for HCC PVTT. Third, this study was conducted in Korea, where HBV infection is the predominant etiology of HCC. Consequently, these findings may not be directly applicable to populations with HCC of different etiologies.
- In conclusion, LR and thrombectomy for patients with HCC PVTT Vp3 or Vp4 may offer the advantage of preventing acute portal occlusion caused by tumor thrombus and can provide acceptable survival benefits. This invasive surgical procedure appears to be a safe and effective treatment modality for selective patients with HCC PVTT Vp3 or Vp4. However, emphasizing the significance of patient selection and meticulous evaluation is crucial in determining the appropriateness of this approach. Further research is essential to validate these findings across diverse patient populations and settings.
DISCUSSION
-
Conflict of Interest
The authors have no conflicts of interest to disclose.
-
Ethics Statement
The study was approved by the Samsung Medical Center Ethics Committee (SMC-2023-11-038) and complied with the Declaration of Helsinki. The Institutional Review Board waived the need for patient consent because of the retrospective nature of this observational study and its use of data from patient medical records.
-
Funding Statement
This research was supported by the Basic Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2023R1A2 C2005946). The funding source had no role in the study design, collection, analysis, or interpretation of the data. This study was supported by the Scientific Research Fund of the Korean Liver Cancer Association (2022).
-
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical regulations.
-
Author Contribution
Conceptualization: JK
Data curation: ML, JK, JR, GSC, JWJ
Formal analysis: ML, JK
Funding acquisition: JK, JR
Methodology: ML, JK
Project administration: JK, GSC, JWJ
Writing original draft: ML, JK
Writing review & editing: JK, JR, GSC, JWJ
Approval of final manuscript: all authors
Article information
Values are presented as number (%) or median (range).
BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; NBNC, non-B, non-C hepatitis; AFP, alpha-fetoprotein; PIVKA-II, prothrombin induced by vitamin K absence II; ICG-R15, indocyanine green retention test at 15 minutes; ALBI, albumin-bilirubin grade; TACE, transarterial chemoembolization, RT, radiation therapy.
OR, odds ratio; CI, confidence interval; BMI, body mass index; PVTT, portal vein tumor thrombosis; TACE, transarterial chemoembolization; WBC, white blood cells; NLR, neutrophil-lymphocyte ratio; NMR, neutrophil-monocyte ratio; AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkaline phosphatase; ALBI, albumin-bilirubin grade; CRP, C-reactive protein; AFP, alpha-fetoprotein; PIVKA-II, prothrombin induced by vitamin K absence II; ICG-R15, indocyanine green retention test at 15 minutes; RBC, red blood cell; APRI, aspartate transaminase to platelet ratio index; MoRAL, the models for tumor recurrence after liver transplantation.
OR, odds ratio; CI, confidence interval; BMI, body mass index; PVTT, portal vein tumor thrombosis; TACE, transarterial chemoembolization; WBC, white blood cells; NLR, neutrophil-lymphocyte ratio; NMR, neutrophil-monocyte ratio; AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkaline phosphatase; ALBI, albumin-bilirubin grade; CRP, C-reactive protein; AFP, alpha-fetoprotein; PIVKA-II, prothrombin induced by vitamin K absence II; ICG-R15, indocyanine green retention test at 15 minutes; RBC, red blood cell; APRI, aspartate transaminase to platelet ratio index; MoRAL, the models for tumor recurrence after liver transplantation.
- 1. Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology 2001;33:1080−1086.ArticlePubMed
- 2. Villa E, Moles A, Ferretti I, Buttafoco P, Grottola A, Del Buono M, et al. Natural history of inoperable hepatocellular carcinoma: estrogen receptors’ status in the tumor is the strongest prognostic factor for survival. Hepatology 2000;32:233−238.ArticlePubMed
- 3. Tarantino L, Busto G, Nasto A, Fristachi R, Cacace L, Talamo M, et al. Percutaneous electrochemotherapy in the treatment of portal vein tumor thrombosis at hepatic hilum in patients with hepatocellular carcinoma in cirrhosis: a feasibility study. World J Gastroenterol 2017;23:906−918.ArticlePubMedPMC
- 4. Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821−829.ArticlePubMed
- 5. Korean Liver Cancer Association (KLCA), National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. J Liver Cancer 2023;23:1−120.ArticlePubMedPMCPDF
- 6. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894−1905.ArticlePubMed
- 7. Yang J, Kim JM, Rhu J, Choi GS, Kwon CHD, Joh JW. surgical resection is preferred in selected solitary hepatocellular carcinoma with portal vein tumor thrombosis. Dig Surg 2022;39:42−50.ArticlePubMedPDF
- 8. Kim JM, Kwon CH, Joh JW, Ko JS, Park JB, Lee JH, et al. C-reactive protein may be a prognostic factor in hepatocellular carcinoma with malignant portal vein invasion. World J Surg Oncol 2013;11:92. ArticlePubMedPMCPDF
- 9. Chen XP, Qiu FZ, Wu ZD, Zhang ZW, Huang ZY, Chen YF, et al. Effects of location and extension of portal vein tumor thrombus on long-term outcomes of surgical treatment for hepatocellular carcinoma. Ann Surg Oncol 2006;13:940−946.ArticlePubMedPDF
- 10. Ikai I, Yamamoto Y, Yamamoto N, Terajima H, Hatano E, Shimahara Y, et al. Results of hepatic resection for hepatocellular carcinoma invading major portal and/or hepatic veins. Surg Oncol Clin N Am 2003;12:65−75. ix.ArticlePubMed
- 11. Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954;7:462−503.ArticlePubMed
- 12. Kim JM, Rhu J, Ha SY, Choi GS, Kwon CHD, Kim G, et al. Realization of improved outcomes following liver resection in hepatocellular carcinoma patients aged 75 years and older. Ann Surg Treat Res 2021;101:257−265.ArticlePubMedPMCPDF
- 13. Yun SO, Kim JM, Rhu J, Choi GS, Joh JW. Fibrosis-4 index, a predictor for prognosis of hepatocellular carcinoma patients after curative hepatectomy even in hepatitis B virus dominant populations. Ann Surg Treat Res 2023;104:195−204.ArticlePubMedPMCPDF
- 14. Cerrito L, Annicchiarico BE, Iezzi R, Gasbarrini A, Pompili M, Ponziani FR. Treatment of hepatocellular carcinoma in patients with portal vein tumor thrombosis: beyond the known frontiers. World J Gastroenterol 2019;25:4360−4382.ArticlePubMedPMC
- 15. Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol 2018;4:661−669.ArticlePubMedPMC
- 16. Finn RS, Zhu AX, Farah W, Almasri J, Zaiem F, Prokop LJ, et al. Therapies for advanced stage hepatocellular carcinoma with macrovascular invasion or metastatic disease: a systematic review and meta-analysis. Hepatology 2018;67:422−435.ArticlePubMedPDF
- 17. Yang Z, Zou R, Zheng Y, Qiu J, Shen J, Liao Y, et al. Lipiodol deposition in portal vein tumour thrombus predicts treatment outcome in HCC patients after transarterial chemoembolisation. Eur Radiol 2019;29:5752−5762.ArticlePubMedPDF
- 18. Hatano E, Uemoto S, Yamaue H, Yamamoto M; Japanese Society of Hepato-Biliary-Pancreatic Surgery. Significance of hepatic resection and adjuvant hepatic arterial infusion chemotherapy for hepatocellular carcinoma with portal vein tumor thrombus in the first branch of portal vein and the main portal trunk: a project study for hepatic surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2018;25:395−402.ArticlePubMedPDF
- 19. Inoue Y, Hasegawa K, Ishizawa T, Aoki T, Sano K, Beck Y, et al. Is there any difference in survival according to the portal tumor thrombectomy method in patients with hepatocellular carcinoma? Surgery 2009;145:9−19.ArticlePubMed
- 20. Kondo K, Chijiiwa K, Kai M, Otani K, Nagaike K, Ohuchida J, et al. Surgical strategy for hepatocellular carcinoma patients with portal vein tumor thrombus based on prognostic factors. J Gastrointest Surg 2009;13:1078−1083.ArticlePubMedPDF
- 21. Han SR, Kim JM, Choi GS, Park JB, Kwon CH, Kim SJ, et al. Protrusion of hepatocellular carcinoma is a predictor of early recurrence in hepatectomy patients after spontaneous rupture. Ann Surg Treat Res 2016;91:17−22.ArticlePubMedPMCPDF
- 22. Kim DS, Kim BW, Hatano E, Hwang S, Hasegawa K, Kudo A, et al. Surgical outcomes of hepatocellular carcinoma with bile duct tumor thrombus: a Korea-Japan multicenter study. Ann Surg 2020;271:913−921.ArticlePubMed
- 23. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907−1917.ArticlePubMed
- 24. Matsumata T, Kanematsu T, Takenaka K, Yoshida Y, Nishizaki T, Sugimachi K. Patterns of intrahepatic recurrence after curative resection of hepatocellular carcinoma. Hepatology 1989;9:457−460.ArticlePubMed
- 25. Ban D, Shimada K, Yamamoto Y, Nara S, Esaki M, Sakamoto Y, et al. Efficacy of a hepatectomy and a tumor thrombectomy for hepatocellular carcinoma with tumor thrombus extending to the main portal vein. J Gastrointest Surg 2009;13:1921−1928.ArticlePubMedPDF
- 26. Aapro M, Österborg A, Gascón P, Ludwig H, Beguin Y. Prevalence and management of cancer-related anaemia, iron deficiency and the specific role of i.v. iron. Ann Oncol 2012;23:1954−1962.ArticlePubMed
- 27. Huang P, Liu C, Li B, Zheng Y, Zou R, Huang J, et al. Preoperative mean corpuscular hemoglobin affecting long-term outcomes of hepatectomized patients with hepatocellular carcinoma. Mol Clin Oncol 2016;4:229−236.ArticlePubMedPMC
- 28. Trotter JF, Cohn A, Grant R. Erythrocytosis in a patient with hepatocellular carcinoma. J Clin Gastroenterol 2002;35:365−366.ArticlePubMed
- 29. Korean Liver Cancer Association (KLCA), National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol 2022;28:583−705.ArticlePubMedPMCPDF
- 30. Lee S, Song SK, Bae B, Park Y. Comparing efficacies of different treatment regimens in patients with hepatocellular carcinoma accompanied by portal vein tumor thrombus using network meta-analysis. Ann Surg Treat Res 2022;103:280−289.ArticlePubMedPMCPDF
- 31. Cho Y, Choi JW, Kwon H, Kim KY, Lee BC, Chu HH, et al. Transarterial chemoembolization for hepatocellular carcinoma: 2023 expert consensus-based practical recommendations of the Korean Liver Cancer Association. J Liver Cancer 2023;23:241−261.ArticlePubMedPMCPDF
- 32. Wei Z, Zhao J, Bi X, Zhang Y, Zhou J, Li Z, et al. Neoadjuvant radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a systematic review. Hepatobiliary Surg Nutr 2022;11:709−717.ArticlePubMedPMC
- 33. Huang DQ, Tran A, Tan EX, Nerurkar SN, Teh R, Teng MLP, et al. Characteristics and outcomes of hepatocellular carcinoma patients with macrovascular invasion following surgical resection: a meta-analysis of 40 studies and 8,218 patients. Hepatobiliary Surg Nutr 2022;11:848−860.ArticlePubMedPMC
- 34. Jin S, Choi WM, Shim JH, Lee D, Kim KM, Lim YS, et al. Subclassification of advanced-stage hepatocellular carcinoma with macrovascular invasion: combined transarterial chemoembolization and radiotherapy as an alternative first-line treatment. J Liver Cancer 2023;23:177−188.ArticlePubMedPMCPDF
- 35. Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol 2019;37:2141−2151.ArticlePubMedPMC
References
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation
- Download Citation
- Close
- Related articles
-
- Comparison of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma with portal vein tumor thrombosis
- Metastatic papillary renal cell carcinoma with portal vein tumor thrombosis confirmed on blind liver biopsy
- Favorable response of hepatocellular carcinoma with portal vein tumor thrombosis after radiotherapy combined with atezolizumab plus bevacizumab
- Radiologic features of hepatocellular carcinoma related to prognosis
- Is multidisciplinary treatment effective for hepatocellular carcinoma with portal vein tumor thrombus?

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION


 Follow JLC on Twitter
Follow JLC on Twitter