Articles
- Page Path
- HOME > J Liver Cancer > Volume 23(1); 2023 > Article
-
Case Report
Favorable response of hepatocellular carcinoma with portal vein tumor thrombosis after radiotherapy combined with atezolizumab plus bevacizumab -
Yong Tae Kim
 , Jina Kim
, Jina Kim , Jinsil Seong
, Jinsil Seong
-
Journal of Liver Cancer 2023;23(1):225-229.
DOI: https://doi.org/10.17998/jlc.2023.02.27
Published online: March 16, 2023
Department of Radiation Oncology, Yonsei Cancer Center, Heavy Ion Therapy Research Institute, Yonsei University College of Medicine, Seoul, Korea
-
Corresponding author: Jinsil Seong, Department of Radiation Oncology, Yonsei Cancer Center, Heavy Ion Therapy Research Institute, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea
Tel. +82-2-2228-8111, Fax. +82-2-2227-7823 E-mail: JSSEONG@yuhs.ac
© 2023 The Korean Liver Cancer Association.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,388 Views
- 74 Downloads
- 2 Citations
Abstract
- Recently, the superiority of atezolizumab plus bevacizumab (AteBeva) over sorafenib was proven in the IMbrave150 trial, and AteBeva became the first-line systemic treatment for untreated, unresectable hepatocellular carcinoma (HCC). While the results are encouraging, more than half of patients with advanced HCC are still being treated in a palliative setting. Radiotherapy (RT) is known to induce immunogenic effects that may enhance the therapeutic efficacy of immune checkpoint inhibitors. Herein, we report the case of a patient with advanced HCC with massive portal vein tumor thrombosis treated with a combination of RT and AteBeva, who showed near complete response in tumor thrombosis and favorable response to HCC. Although this is a rare case, it shows the importance of reducing the tumor burden via RT to combination immunotherapy in patients with advanced HCC.
- As a result of several phase II trials, immunotherapy has been presented as an alternative treatment option for patients with advanced hepatocellular carcinoma (HCC) who have failed standard treatments, such as sorafenib and lenvatinib.1,2 Moreover, the recently published results of the IMbrave150 trial have caused a paradigm shift in systemic treatment.3 However, treatment with immune checkpoint inhibitors (ICI) alone shows a low objective response rate (ORR) of 15–20%, and they remain in the palliative setting.1,2 Therefore, there is a growing need for novel treatment modalities that can improve oncologic outcomes in combination with immunotherapy.
- Radiotherapy (RT) has been conventionally used for local tumor control. However, it is also known to have immune-modulating effects, such as direct tumor cell killing through DNA double-strand breakage, tumor microenvironment (TME) alteration, and tumor-associated antigen (TAA) release, which are expected to improve the therapeutic effect of ICI.4,5 Substantial treatment outcomes of ICI combined with RT have been reported in various solid tumors, but there are not much clinical data on HCC.
- Here, we report the case of a patient with advanced HCC with massive portal vein tumor thrombosis (PVTT) treated with a combination of RT and atezolizumab plus bevacizumab (AteBeva), who showed near complete response (CR) in tumor thrombosis and favorable response to HCC. This case report was described according to the CARE guidelines available at https://www.care-statement.org/. This study was approved by the Institutional Review Board (IRB number: 4-2022-1437), and the requirement for informed consent from patients was waived because of its retrospective nature.
INTRODUCTION
- A 57-year-old man referred from a local hospital complained of right upper quadrant discomfort and liver function test (LFT) abnormalities. The patient had no history of viral hepatitis or underlying liver disease. He had no family history of liver cancer but had a history of heavy alcohol consumption (28.6 g/day, three times a week).
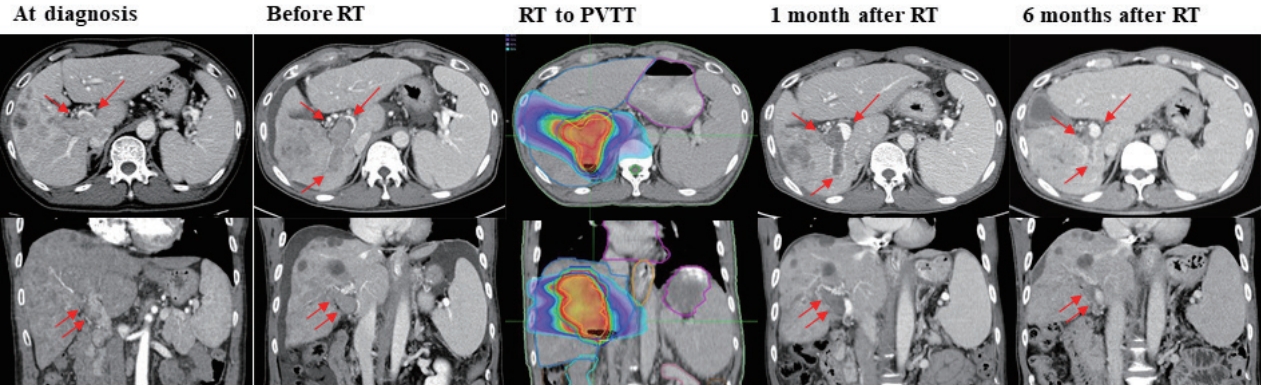
- Initial abdominal dynamic computed tomography (CT) and magnetic resonance imaging showed an infiltrative mass involving the right lobe of the liver from the dome, measuring approximately 17 cm in the longest dimension, and PVTT was present from the right portal vein to the main portal vein (Fig. 1). Additionally, metastatic lymph nodes were observed in the hepatoduodenal, portocaval, and aortocaval areas. No viable lesions were observed in the left lobe of the liver.
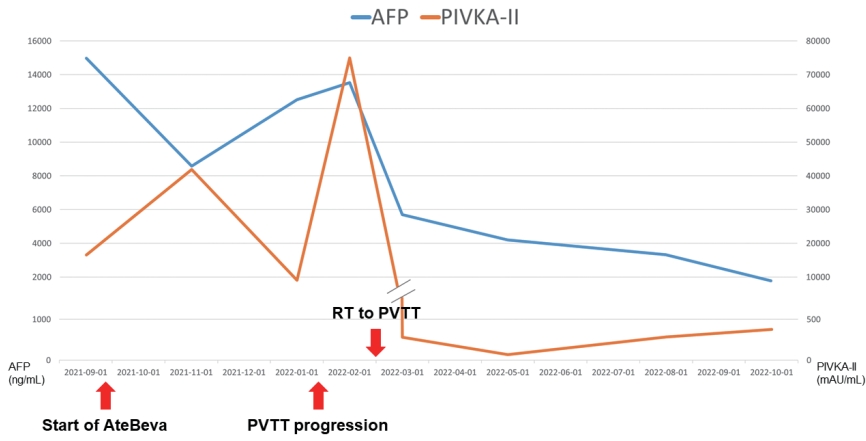
- The initial laboratory findings were as follows: white blood cell count, 5.58×103/µL; hemoglobin level, 14.1 g/dL, platelet count, 124×103/µL. LFT revealed a serum bilirubin level of 0.9 mg/dL, an albumin level of 4.6 g/dL, a prothrombin time international normalized ratio of 0.94, an aspartate aminotransferase of 66 IU/L, and an alanine aminotransferase of 18 IU/L. Additionally, the serum alpha-fetoprotein (AFP) level was 14,979.40 ng/mL, while the protein induced by vitamin K absence or antagonist-II (PIVKA-II) level was 16,578 mAU/mL.
- On the chest CT scan, metastasis was suspected because the highest mediastinum, prevascular, bilateral internal mammary chain, and right cardiophrenic lymph nodes were enlarged, and the lesions showed intense F-18 fluorodeoxyglucose uptake on positron emission tomography-CT. A liver biopsy was performed, and the pathology confirmed moderately differentiated HCC.
- This patient was staged as stage C according to the Barcelona Clinic Liver Cancer (BCLC) criteria and stage IVB (T4N1M1) according to the modified Union for International Cancer Control. Liver function was well preserved with a Child-TurcottePugh class of A5, and the Eastern Cooperative Oncology Group performance score was 0. There was no varix on the initial esophagogastroduodenoscopy.
- We started atezolizumab (1,200 mg) plus bevacizumab (15 mg/kg) combination therapy at 3-week intervals. After every three cycles of AteBeva administration, a follow-up CT was performed. On the first response evaluation after initiation of treatment, abdominopelvic CT showed a stable status of the primary infiltrative tumor, and chest CT showed shrinkage of mediastinal lymph nodes. The serum AFP level decreased to 8,584.60 ng/mL, while the PIVKA-II level increased to 41,910 mAU/mL.
- After the sixth cycle of AteBeva, follow-up liver CT showed progressive PVTT. The extent of the tumor thrombosis increased peripherally to the right posterior segment of the portal vein and further to the central portion of the main trunk. The expansile portion of the tumor thrombosis also increased in size. Additionally, the AFP increased to 12,514.10 ng/mL, and PIVKA-II decreased to 9,072 mAU/mL.
- The patient was referred to the Department of Radiation Oncology for local control of progressive tumor thrombosis. RT was delivered only to the PVTT, including the main portal vein and the right portal vein to the first branch. RT was scheduled to be delivered between the seventh and eighth cycles of AteBeva with 50 Gy in 10 fractions. The tumor markers identified during RT were as follows: AFP 13,536.50 ng/mL, PIVKA-II >75,000 mAU/mL. No acute toxicity or radiation-induced liver dysfunction was reported during treatment, and the patient tolerated the treatment well.
- On liver CT performed 1 month after the completion of RT, tumor enhancement in the PVTT decreased, and an early response with decreased tumor extent was observed. AFP decreased from 13,536.5 ng/mL to 5,694.7 ng/mL, and PIVKA-II markedly decreased from >75,000 mAU/mL to 281 mAU/mL. Meanwhile, systemic therapy with AteBeva was maintained, and at 6 months after RT, CT evaluation showed almost CR of tumor thrombosis in the right portal vein and main portal vein. In addition, the primary tumor showed a remarkable response, as evaluated by the sum of the lengths of the infiltrative tumor, which was initially 17 cm, but is now 8.7 cm. On follow-up chest CT, the mediastinal lymph nodes that showed a partial response from sixth cycle continued to be small and stable. The clinical course of HCC and tumor markers during follow-up are shown in Figs. 1, 2.
- After the 18th administration of AteBeva, a newly appearing HCC was observed in the left lobe of the liver (segment III), whereas the rest of the lesions remained stable. After a multidisciplinary team discussion, the patient is currently scheduled to receive additional salvage RT for this new lesion.
CASE REPORT
- In this study, we report a case of advanced HCC that was treated with a combination of RT and AteBeva. The patient was diagnosed with polymetastatic HCC with massive PVTT and extrahepatic metastasis, and AteBeva was administered as the current standard systemic treatment. After the second response evaluation, PVTT showed disease progression, and hypofractionated RT was delivered as a palliative aim. During serial follow-up, the enhancement and extent of tumor thrombosis gradually decreased, and finally, near CR of the irradiated PVTT and partial response of the non-irradiated primary mass were observed. During the 18th cycle of AteBeva, 12.7 months of response duration was achieved, and we are currently planning salvage RT for the newly developed lesion.
- Recently, the superiority of AteBeva over sorafenib was proven by the IMbrave150 trial, and AteBeva became the first-line systemic treatment for untreated unresectable HCC. The 1-year estimated overall survival (OS) rate was 67.2% (95% confidence interval [CI], 61.3–73.1) in the AteBeva group and 54.6% (95% CI, 45.2–64.0) in the sorafenib group.3 Subsequent report with a longer follow-up (12 months of additional follow-up) data also showed the superiority of AteBeva over sorafenib, with median OS of 19.2 and 13.4 months in the AteBeva and sorafenib groups, respectively (hazard ratio, 0.66; 95% CI, 0.52–0.85; P=0.0009). The confirmed ORR of AteBeva was 29.8% (per RECIST 1.1), with more patients achieving CR (7.7%).6 Another study investigated the rea-lworld efficacy of AteBeva in the Korean population, with an ORR of 24.0% and a median progression-free survival of 6.5 months.7 While the results are encouraging, more than half of patients with advanced HCC still have poor prognosis.
- In the immunotherapy era, the rationale for combining atezolizumab (an ICI) and bevacizumab (an anti-vascular endothelial growth factor [VEGF]) is based on the expectation of enhancing the efficacy of anti-programmed cell death protein 1 and anti-programmed death-ligand 1 by reducing VEGF-mediated immunosuppression within the tumor and its microenvironment and by promoting T-cell infiltration into tumor cells.3 RT not only induces direct tumor cell death in the irradiated area but also promotes immunogenic tumor cell death through changes in the TME and the release of TAAs.4,5 Thus, enhanced oncologic outcomes are expected from the combination of ICI and RT for HCC.
- In this study, we report a successful tumor response of AteBeva in combination with RT to the PVTT. PVTT, the most common form of macrovascular invasion in HCC, is associated with tumor invasiveness and may adversely affect the maintenance of systemic treatment owing to liver function deterioration caused by portal hypertension. Many previous studies have reported the efficacy of RT in controlling PVTT.8-10 With modern high-precision intensity-modulated RT techniques, local control of PVTT is possible without causing RT-related liver toxicity. In addition, the tumor burden reduction achieved with RT may have enhanced ICI efficacy. It is known that the larger the tumor size, the less effective the ICI treatment and the poorer the prognosis.11
- In conclusion, we report successful tumor control of HCC with a combination of RT to PVTT with AteBeva in patients with advanced-stage HCC. Massive PVTT (Vp4) showed CR, and the non-irradiated tumor was also well controlled. While the current guidelines for BCLC stage C HCC are mainly focused on systemic therapy, this case demonstrates the importance of adding RT to combination immunotherapy to reduce the tumor burden. However, further studies are needed to determine whether adding RT is more advantageous than systemic immunotherapy alone.
DISCUSSION
-
Conflicts of Interest
The authors have no conflicts of interest to disclose.
-
Ethics Statement
This study was approved by the Institutional Review Board of Severance Hospital (IRB number: 4-2022-1437), and the requirement for informed consent from patients was waived because of its retrospective nature.
-
Funding Statement
None.
-
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed for this case report.
-
Author Contribution
Conceptualization: JS
Data curation: YTK, JK
Methodology: JS
Project administration: JS
Writing original draft: YTK
Writing review & editing: JK, JS
Approval of final manuscript: all authors
Article information
- 1. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492−2502.ArticlePubMedPMC
- 2. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a nonrandomised, open-label phase 2 trial. Lancet Oncol 2018;19:940−952.PubMed
- 3. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894−1905.ArticlePubMed
- 4. Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015;16:e498−e509.ArticlePubMed
- 5. Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol 2016;13:516−524.ArticlePubMedPMCPDF
- 6. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol 2021;39 Suppl 3:267. Article
- 7. Cheon J, Yoo C, Hong JY, Kim HS, Lee DW, Lee MA, et al. Efficacy and safety of atezolizumab plus bevacizumab in Korean patients with advanced hepatocellular carcinoma. Liver Int 2022;42:674−681.ArticlePubMedPDF
- 8. Rim CH, Yang DS, Park YJ, Yoon WS, Lee JA, Kim CY. Effectiveness of high-dose three-dimensional conformal radiotherapy in hepatocellular carcinoma with portal vein thrombosis. Jpn J Clin Oncol 2012;42:721−729.ArticlePubMed
- 9. Su F, Chen KH, Liang ZG, Wu CH, Li L, Qu S, et al. Comparison of three-dimensional conformal radiotherapy and hepatic resection in hepatocellular carcinoma with portal vein tumor thrombus. Cancer Med 2018;7:4387−4395.ArticlePubMedPMCPDF
- 10. Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol 2019;37:2141−2151.ArticlePubMedPMC
- 11. Dall'Olio FG, Marabelle A, Caramella C, Garcia C, Aldea M, Chaput N, et al. Tumour burden and efficacy of immune-checkpoint inhibitors. Nat Rev Clin Oncol 2022;19:75−90.ArticlePubMedPDF
References
Figure & Data
References
Citations

- Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab
Tae Hyun Kim, Bo Hyun Kim, Yu Ri Cho, Young-Hwan Koh, Joong-Won Park
Journal of Liver Cancer.2023; 23(2): 330. CrossRef - Carbon Ion Radiotherapy in the Treatment of Hepatocellular Carcinoma
Hwa Kyung Byun, Changhwan Kim, Jinsil Seong
Clinical and Molecular Hepatology.2023; 29(4): 945. CrossRef
 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation
- Download Citation
- Close
- Related articles
-
- Liver resection in selective hepatocellular carcinoma with Vp3 or Vp4 portal vein tumor thrombosis improves prognosis
- Comparison of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma with portal vein tumor thrombosis
- Metastatic papillary renal cell carcinoma with portal vein tumor thrombosis confirmed on blind liver biopsy
- Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab
- A case of nearly complete response in hepatocellular carcinoma with disseminated lung metastasis by combination therapy of nivolumab and ipilimumab after treatment failure of atezolizumab plus bevacizumab

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION


 Follow JLC on Twitter
Follow JLC on Twitter