Search
- Page Path
- HOME > Search
Original Article
- Comparison of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma with portal vein tumor thrombosis
- Jeayeon Park, Yun Bin Lee, Yunmi Ko, Youngsu Park, Hyunjae Shin, Moon Haeng Hur, Min Kyung Park, Dae-Won Lee, Eun Ju Cho, Kyung-Hun Lee, Jeong-Hoon Lee, Su Jong Yu, Tae-Yong Kim, Yoon Jun Kim, Tae-You Kim, Jung-Hwan Yoon
- J Liver Cancer. 2024;24(1):81-91. Published online January 19, 2024
- DOI: https://doi.org/10.17998/jlc.2023.12.25
- 2,107 Views
- 167 Downloads
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background/Aim
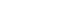
Atezolizumab plus bevacizumab and lenvatinib are currently available as first-line therapy for the treatment of unresectable hepatocellular carcinoma (HCC). However, comparative efficacy studies are still limited. This study aimed to investigate the effectiveness of these treatments in HCC patients with portal vein tumor thrombosis (PVTT).
Methods
We retrospectively included patients who received either atezolizumab plus bevacizumab or lenvatinib as first-line systemic therapy for HCC with PVTT. Primary endpoint was overall survival (OS), and secondary endpoints included progressionfree survival (PFS) and disease control rate (DCR) determined by response evaluation criteria in solid tumors, version 1.1.
Results
A total of 52 patients were included: 30 received atezolizumab plus bevacizumab and 22 received lenvatinib. The median follow-up duration was 6.4 months (interquartile range, 3.9-9.8). The median OS was 10.8 months (95% confidence interval [CI], 5.7 to not estimated) with atezolizumab plus bevacizumab and 5.8 months (95% CI, 4.8 to not estimated) with lenvatinib (P=0.26 by log-rank test). There was no statistically significant difference in OS (adjusted hazard ratio [aHR], 0.71; 95% CI, 0.34-1.49; P=0.37). The median PFS was similar (P=0.63 by log-rank test), with 4.1 months (95% CI, 3.3-7.7) for atezolizumab plus bevacizumab and 4.3 months (95% CI, 2.6-5.8) for lenvatinib (aHR, 0.93; 95% CI, 0.51-1.69; P=0.80). HRs were similar after inverse probability treatment weighting. The DCRs were 23.3% and 18.2% in patients receiving atezolizumab plus bevacizumab and lenvatinib, respectively (P=0.74).
Conclusion
The effectiveness of atezolizumab plus bevacizumab and lenvatinib was comparable for the treatment of HCC with PVTT.

Review Articles
- Complications of immunotherapy in advanced hepatocellular carcinoma
- Young-Gi Song, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
- J Liver Cancer. 2024;24(1):9-16. Published online November 29, 2023
- DOI: https://doi.org/10.17998/jlc.2023.11.21
- 2,115 Views
- 122 Downloads
- 2 Citations
-
 Abstract
Abstract
 PDF
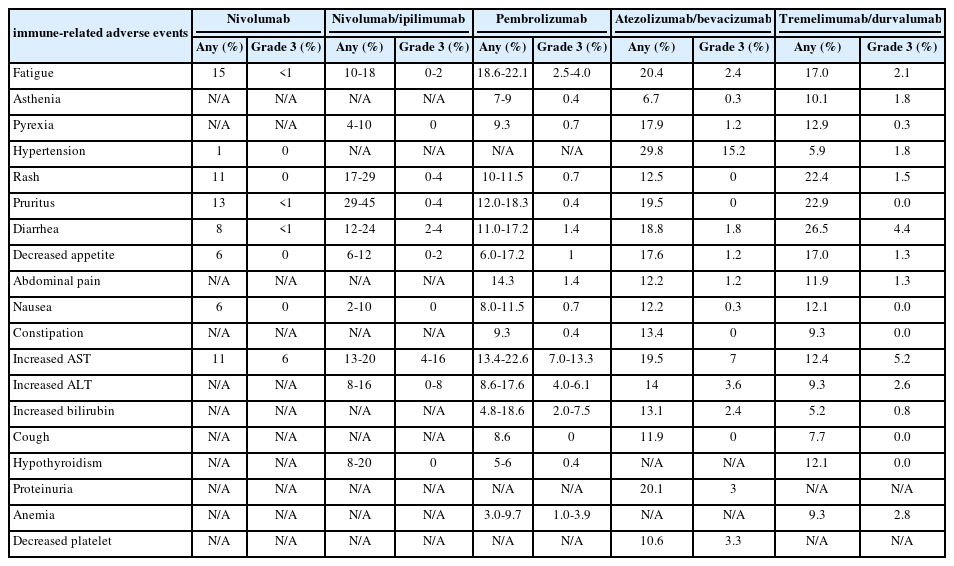
PDF - Immune checkpoint inhibitors (ICIs) are highly effective in cancer treatment. However, the risks associated with the treatment must be carefully balanced against the therapeutic benefits. Immune-related adverse events (irAEs) are generally unpredictable and may persist over an extended period. In this review, we analyzed common irAEs reported in highly cited original articles and systematic reviews. The prevalent adverse reactions include fatigue, pyrexia, rash, pruritus, diarrhea, decreased appetite, nausea, abdominal pain, constipation, hepatitis, and hypothyroidism. Therefore, it is crucial to conduct evaluations not only of gastrointestinal organs but also of cardiac, neurologic, endocrine (including the frequently affected thyroid), and ophthalmic systems before commencing ICIs. This review further explores commonly reported types of irAEs, specific irAEs associated with each ICI agent, rare yet potentially fatal irAEs, and available treatment options for managing them.
-
Citations
Citations to this article as recorded by- Intrahepatic IgA complex induces polarization of cancer-associated fibroblasts to matrix phenotypes in the tumor microenvironment of HCC
Jong Geun Park, Pu Reun Roh, Min Woo Kang, Sung Woo Cho, Suhyun Hwangbo, Hae Deok Jung, Hyun Uk Kim, Ji Hoon Kim, Jae-Sung Yoo, Ji Won Han, Jeong Won Jang, Jong Young Choi, Seung Kew Yoon, Young Kyoung You, Ho Joong Choi, Jae Yong Ryu, Pil Soo Sung
Hepatology.2024;[Epub] CrossRef - Risk of Bleeding in Hepatocellular Carcinoma Patients Treated with Atezolizumab/Bevacizumab: A Systematic Review and Meta-Analysis
Young-Gi Song, Kyeong-Min Yeom, Eun Ae Jung, Sang Gyune Kim, Young Seok Kim, Jeong-Ju Yoo
Liver Cancer.2024; : 1. CrossRef
- Intrahepatic IgA complex induces polarization of cancer-associated fibroblasts to matrix phenotypes in the tumor microenvironment of HCC

- A multidisciplinary approach with immunotherapies for advanced hepatocellular carcinoma
- Yu Rim Lee
- J Liver Cancer. 2023;23(2):316-329. Published online September 22, 2023
- DOI: https://doi.org/10.17998/jlc.2023.09.04

- 2,032 Views
- 115 Downloads
- 4 Citations
-
 Abstract
Abstract
 PDF
PDF - Hepatocellular carcinoma (HCC) is a highly aggressive disease that is usually diagnosed at an advanced stage. Advanced HCC has limited treatment options and often has a poor prognosis. For the past decade, tyrosine kinase inhibitors have been the only treatments approved for advanced HCC that have shown overall survival (OS) benefits; however, but their clinical efficacy has been limited. Recent trials have demonstrated promising advancements in survival outcomes through immunotherapy-based treatments, such as combinations of immune checkpoint inhibitors (ICIs) with other ICIs, antiangiogenic drugs, and locoregional therapies. The atezolizumab-bevacizumab and durvalumab-tremelimumab (STRIDE) regimen has significantly improved survival rates as a first-line treatment and has become the new standard of care. Therefore, combined treatments for advanced HCC can result in better treatment outcomes owing to their synergistic effects, which requires a multidisciplinary approach. Ongoing studies are examining other therapeutic innovations that can improve disease control and OS rates. Despite improvements in the treatment of advanced HCC, further studies on the optimal treatment selection and sequences, biomarker identification, combination approaches with other therapies, and development of novel immunotherapy agents are required. This review presents the current treatment options and clinical data of the ICI-based combination immunotherapies for advanced HCC from a multidisciplinary perspective.
-
Citations
Citations to this article as recorded by- Reduced-Dose or Discontinuation of Bevacizumab Might Be Considered after Variceal Bleeding in Patients with Hepatocellular Carcinoma Receiving Atezolizumab/Bevacizumab: Case Reports
Kyeong-Min Yeom, Young-Gi Song, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
Medicina.2024; 60(1): 157. CrossRef - Hepatocellular Carcinoma: Advances in Systemic Therapy
Insija Ilyas Selene, Merve Ozen, Reema A. Patel
Seminars in Interventional Radiology.2024; 41(01): 056. CrossRef - Fatal intratumoral hemorrhage in a patient with hepatocellular carcinoma following successful treatment with atezolizumab/bevacizumab: A case report
Kyeong-Hoon Park, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
World Journal of Clinical Cases.2024; 12(22): 5177. CrossRef - Unravelling infiltrating T‐cell heterogeneity in kidney renal clear cell carcinoma: Integrative single‐cell and spatial transcriptomic profiling
Haiqing Chen, Haoyuan Zuo, Jinbang Huang, Jie Liu, Lai Jiang, Chenglu Jiang, Shengke Zhang, Qingwen Hu, Haotian Lai, Bangchao Yin, Guanhu Yang, Gang Mai, Bo Li, Hao Chi
Journal of Cellular and Molecular Medicine.2024;[Epub] CrossRef
- Reduced-Dose or Discontinuation of Bevacizumab Might Be Considered after Variceal Bleeding in Patients with Hepatocellular Carcinoma Receiving Atezolizumab/Bevacizumab: Case Reports

- The role of lenvatinib in the era of immunotherapy of hepatocellular carcinoma
- Matthew Man Pok Lee, Landon Long Chan, Stephen Lam Chan
- J Liver Cancer. 2023;23(2):262-271. Published online August 17, 2023
- DOI: https://doi.org/10.17998/jlc.2023.07.17
- 3,532 Views
- 281 Downloads
- 5 Citations
-
 Abstract
Abstract
 PDF
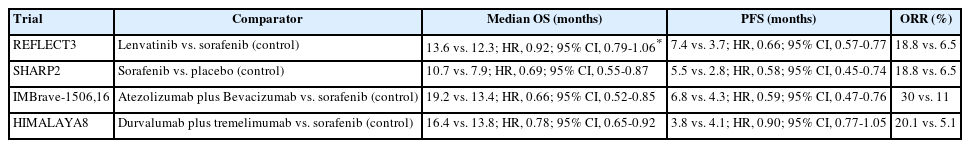
PDF - Hepatocellular carcinoma (HCC) frequently presents as advanced stage with poor prognosis and high mortality. Systemic treatment is the treatment of choice for advanced disease. In 2007, the first multi-kinase inhibitor (MKI) sorafenib was approved and shown to modestly prolong overall survival (OS). The progress of systemic therapy has been slow afterwards until 2018 when lenvatinib, another MKI, was shown to be non-inferior to sorafenib on median OS as the first-line therapy for HCC. Since then, remarkable progress has been achieved on the treatment of advanced HCC, including the development of second-line targeted treatment, including regorafenib, cabozantinib and ramucirumab from 2017 to 2019. A growing focus has been placed on immune checkpoint inhibitors (ICIs) targeting programmed cell death-1 (PD-1), its ligand PD-L1, and cytotoxic T-lymphocyte-associated protein 4. These ICIs have proven their potency in treating HCC as both initial and subsequent line of therapy. At present, both regimens of atezolizumab combined with bevacizumab, as well as the combination of tremelimumab and durvalumab, are recommended as the first-line treatments based on positive phase III clinical trials. With the advancement of ICIs, it is anticipated that the role of MKIs in the treatment of HCC will evolve. In this article, lenvatinib, one of the most commonly used MKIs in HCC, is chosen to be reviewed.
-
Citations
Citations to this article as recorded by- Reduced-Dose or Discontinuation of Bevacizumab Might Be Considered after Variceal Bleeding in Patients with Hepatocellular Carcinoma Receiving Atezolizumab/Bevacizumab: Case Reports
Kyeong-Min Yeom, Young-Gi Song, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
Medicina.2024; 60(1): 157. CrossRef - The Position of Multikinase Inhibitors in the Era of Immune-Checkpoint Inhibitors for Hepatocellular Carcinoma
Beom Kyung Kim
Gut and Liver.2024; 18(1): 3. CrossRef - Fatal intratumoral hemorrhage in a patient with hepatocellular carcinoma following successful treatment with atezolizumab/bevacizumab: A case report
Kyeong-Hoon Park, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
World Journal of Clinical Cases.2024; 12(22): 5177. CrossRef - Small molecule tyrosine kinase inhibitors approved for systemic therapy of advanced hepatocellular carcinoma: recent advances and future perspectives
Jianzhong Liu, Shuai Xia, Baoyi Zhang, Dina Mostafa Mohammed, Xiangliang Yang, Yanhong Zhu, Xinnong Jiang
Discover Oncology.2024;[Epub] CrossRef - Consistent efficacy of hepatic artery infusion chemotherapy irrespective of PD‑L1 positivity in unresectable hepatocellular carcinoma
Ji Kim, Young Kim, Hee-Chul Nam, Chang-Wook Kim, Jae-Sung Yoo, Ji Han, Jeong Jang, Jong Choi, Seung Yoon, Ho Jong Chun, Jung Oh, Suho Kim, Sung Lee, Pil Sung
Oncology Letters.2024;[Epub] CrossRef
- Reduced-Dose or Discontinuation of Bevacizumab Might Be Considered after Variceal Bleeding in Patients with Hepatocellular Carcinoma Receiving Atezolizumab/Bevacizumab: Case Reports

Case Report
- Concurrent transarterial radioembolization and combination atezolizumab/ bevacizumab treatment of infiltrative hepatocellular carcinoma with portal vein tumor thrombosis: a case report
- Min Kyung Park, Su Jong Yu
- J Liver Cancer. 2022;22(1):69-74. Published online March 21, 2022
- DOI: https://doi.org/10.17998/jlc.2022.03.09
- 3,745 Views
- 112 Downloads
- 4 Citations
-
 Abstract
Abstract
 PDF
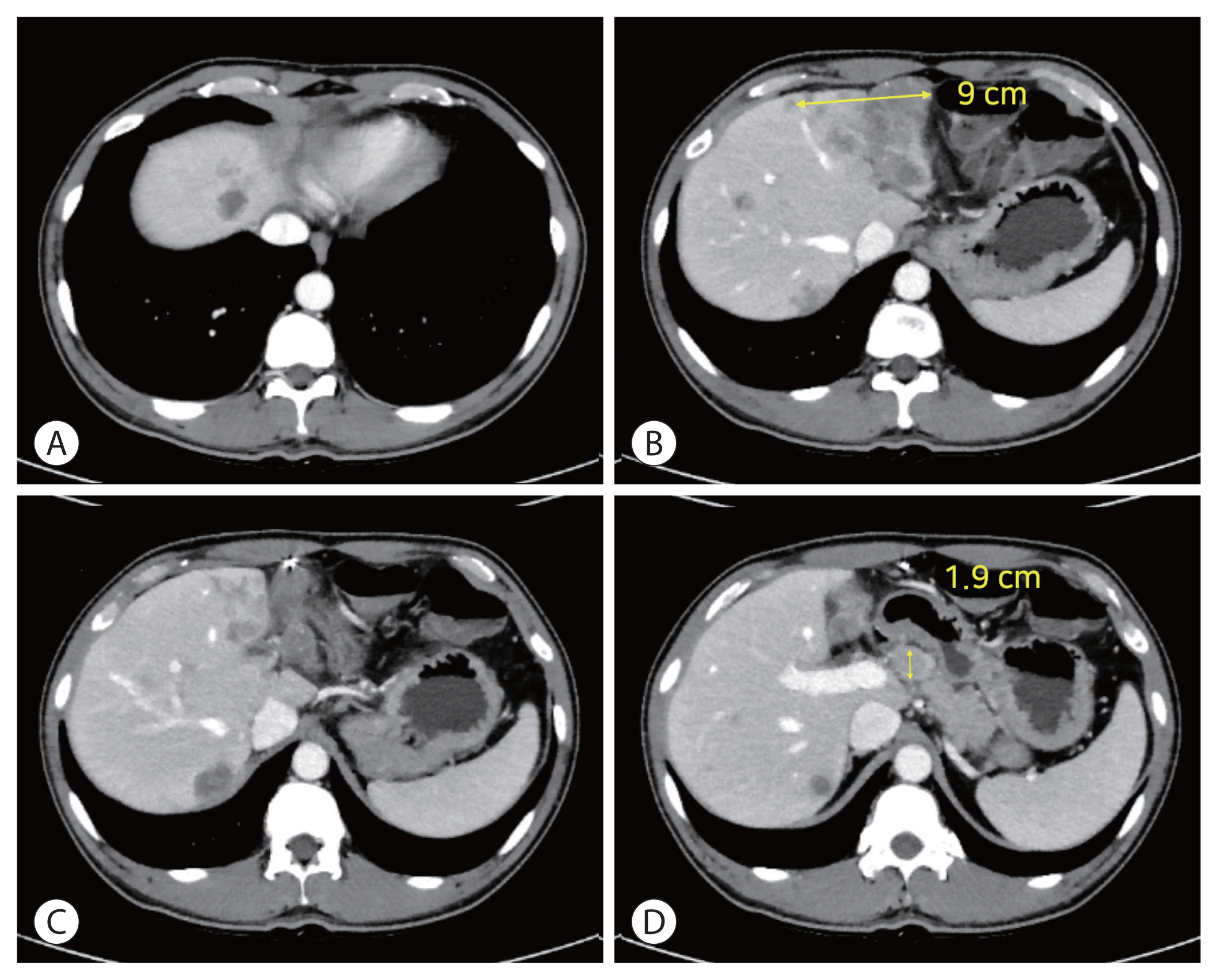
PDF - Treatment options for advanced hepatocellular carcinoma (HCC) have been rapidly evolving. Herein, we describe a patient with advanced HCC and portal vein tumor thrombosis (PVTT) who responded decisively to a multidisciplinary approach. The patient had an ill-defined infiltrative HCC (diffuse subtype), with several intrahepatic metastasis and tumor invasion of left portal vein. Concurrent use of transarterial radioembolization (TARE) and systemic therapeutics (atezolizumab + bevacizumab) ultimately proved successful. There was marked reduction in tumor volume after TARE and an additional three cycles of atezolizumab plus bevacizumab. This concurrent treatment was well tolerated, without adverse events during immunotherapy. The impressive results achieved suggest that concurrent TARE and combination atezolizumab/bevacizumab is a promising treatment approach for advanced HCC with PVTT.
-
Citations
Citations to this article as recorded by- Biologics, Immunotherapies, and Cytotoxic Chemotherapy for Hepatocellular Carcinoma following Current Recommendations by the BCLC: A Review of Agents
Rajangad S. Gurtatta, Sydney E. Whalen, Charles E. Ray
Seminars in Interventional Radiology.2024; 41(01): 084. CrossRef - Combining immunotherapy with transarterial radioembolization
ZeynepCeren Balaban Genc, Efe Soydemır, SevalAy Ersoy, Tunc Ones
Indian Journal of Nuclear Medicine.2023; 38(2): 145. CrossRef - The New Era of Systemic Treatment for Hepatocellular Carcinoma: From the First Line to the Optimal Sequence
Maria Cerreto, Ferdinando Cardone, Lucia Cerrito, Leonardo Stella, Francesco Santopaolo, Maria Pallozzi, Antonio Gasbarrini, Francesca Romana Ponziani
Current Oncology.2023; 30(10): 8774. CrossRef - Is multidisciplinary treatment effective for hepatocellular carcinoma with portal vein tumor thrombus?
Won Hyeok Choe
Journal of Liver Cancer.2022; 22(1): 1. CrossRef
- Biologics, Immunotherapies, and Cytotoxic Chemotherapy for Hepatocellular Carcinoma following Current Recommendations by the BCLC: A Review of Agents

Review Articles
- Advances in immune checkpoint inhibitors for hepatocellular carcinoma
- Ji Won Han, Su-Hyung Park
- J Liver Cancer. 2021;21(2):139-145. Published online September 30, 2021
- DOI: https://doi.org/10.17998/jlc.2021.09.24
- 3,975 Views
- 105 Downloads
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF - Hepatocellular carcinoma (HCC) is the fifth most common cancer, and the second leading cause of cancer-related death worldwide. Although recent advances in immune checkpoint inhibitor-based immunotherapy have initiated a new era for advanced HCC treatment, the majority of HCC patients receiving immune checkpoint blockades do not derive clinical benefit. Thus, there remains an urgent need for novel immunotherapeutic strategies with improved therapeutic efficacy. Here we review recent studies of immune checkpoint blockade in HCC, providing the necessary basis for the rational design of immunotherapy.
-
Citations
Citations to this article as recorded by- Systematic Review of Molecular Targeted Therapies for Adult-Type Diffuse Glioma: An Analysis of Clinical and Laboratory Studies
Logan Muzyka, Nicolas K. Goff, Nikita Choudhary, Michael T. Koltz
International Journal of Molecular Sciences.2023; 24(13): 10456. CrossRef - Integrative analysis of lactylation-related genes and establishment of a novel prognostic signature for hepatocellular carcinoma
Diankui Cai, Xiaoqing Yuan, D. Q. Cai, Ang Li, Sijia Yang, Weibang Yang, Jinxin Duan, Wenfeng Zhuo, Jun Min, Li Peng, Jinxing Wei
Journal of Cancer Research and Clinical Oncology.2023; 149(13): 11517. CrossRef - Editorial on Immune Checkpoint Inhibitors in the Treatment of Hepatocellular Carcinoma
Samantha M Ruff, Timothy M Pawlik
Immunotherapy.2023; 15(16): 1323. CrossRef
- Systematic Review of Molecular Targeted Therapies for Adult-Type Diffuse Glioma: An Analysis of Clinical and Laboratory Studies

- Systemic therapy for advanced hepatocellular carcinoma: consideration for selecting second-line treatment
- Bo Hyun Kim, Joong-Won Park
- J Liver Cancer. 2021;21(2):124-138. Published online September 30, 2021
- DOI: https://doi.org/10.17998/jlc.2021.09.23
- 4,422 Views
- 120 Downloads
- 2 Citations
-
 Abstract
Abstract
 PDF
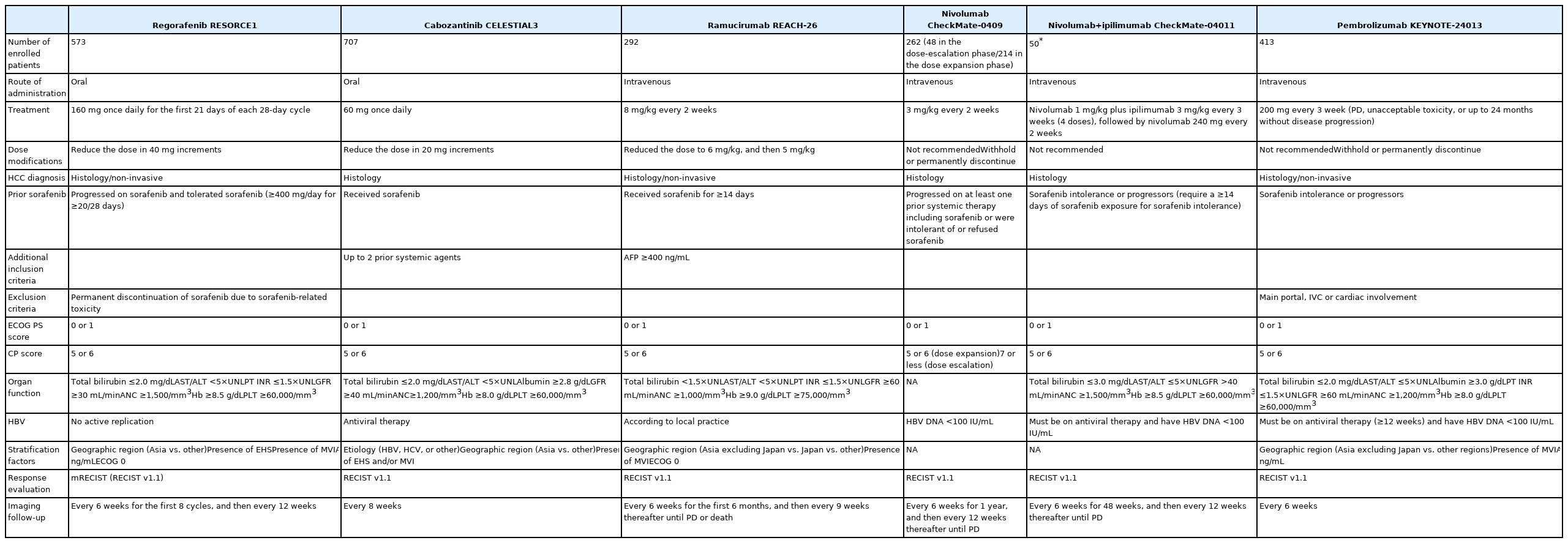
PDF - Several molecular-targeted agents have been tested as first- or second-line therapies for hepatocellular carcinoma (HCC) but failed to improve clinical outcomes; sorafenib has been the only approved systemic agent for treating HCC for almost 10 years. Regorafenib resulted in a significant improvement in overall survival and thus was approved for HCC patients previously treated with sorafenib. Subsequently, cabozantinib and ramucirumab demonstrated superior overall survival compared with placebos in phase III clinical trials. Immune checkpoint inhibitors such as nivolumab with or without ipilimumab and pembrolizumab are also available in some countries for patients who are unresponsive to sorafenib. Some second-line agents are available for patients who are unresponsive to sorafenib; however, little is known about the considerations for selecting appropriate secondline systemic agents. Hence, this study aimed to review the current and future perspectives of second-line systemic agents.
-
Citations
Citations to this article as recorded by- Expression of Peptidyl Arginine Deiminase 2 Is Closely Associated with Recurrence in Patients with Hepatocellular Carcinoma
Sunho Uhm, Yoon Cho, Ji-Young Choe, Ji Park, Min-Jeong Kim, Won-Ho Han, Junyong Lee, Jung Lee, Dong Shin, Jae Soh, Hyun Lim, Ho Kang, Sung-Hoon Moon, Sung-Eun Kim
Diagnostics.2023; 13(4): 659. CrossRef - Expert consensus on the management of adverse events in patients receiving lenvatinib for hepatocellular carcinoma
Bo Hyun Kim, Su Jong Yu, Wonseok Kang, Sung Bum Cho, Soo Young Park, Seung Up Kim, Do Young Kim
Journal of Gastroenterology and Hepatology.2022; 37(3): 428. CrossRef
- Expression of Peptidyl Arginine Deiminase 2 Is Closely Associated with Recurrence in Patients with Hepatocellular Carcinoma

- Deciphering and Reversing Immunosuppressive Cells in the Treatment of Hepatocellular Carcinoma
- Su Jong Yu, Tim F. Greten
- J Liver Cancer. 2020;20(1):1-16. Published online March 31, 2020
- DOI: https://doi.org/10.17998/jlc.20.1.1
- 7,110 Views
- 197 Downloads
- 3 Citations
-
 Abstract
Abstract
 PDF
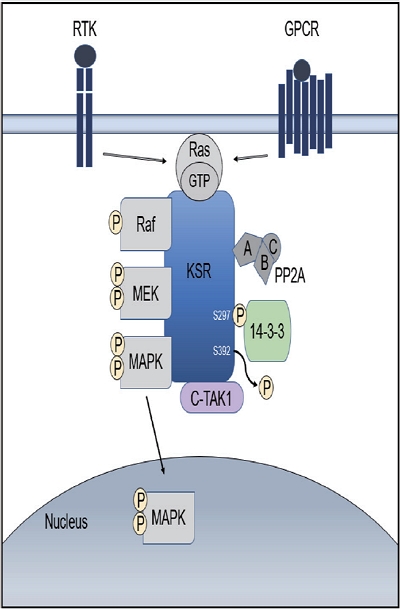
PDF - Use of immune checkpoint inhibitors (ICIs) in hepatocellular carcinoma (HCC) has been partially successful. However, most HCC patients do not respond to immunotherapy. HCC has been shown to induce several immune suppressor mechanisms in patients. These suppressor mechanisms include involvement of myeloid-derived suppressor cells, regulatory T-cells, functionally impaired dendritic cells (DCs), neutrophils, monocytes, and tumor associated macrophages. The accumulation of immunosuppressive cells may lead to an immunosuppressive tumor microenvironment as well as the dense fibrotic stroma which may contribute to immune tolerance. Our laboratory has been investigating different cellular mechanisms of immune suppression in HCC patients. In vitro as well as in vivo studies have demonstrated that abrogation of the suppressor cells enhances or unmasks tumor-specific antitumor immune responses. Two or three effective systemic therapies including ICIs and/or molecular targeted therapies and the addition of innovative combination therapies targeting immune suppressor cells may lead to increased immune recognition with a greater tumor response. We reviewed the literature for the latest research on immune suppressor cells in HCC, and here we provide a comprehensive summary of the recent studies in this field.
-
Citations
Citations to this article as recorded by- Higher Number of Tumor-Infiltrating PD-L1+ Cells Is Related to Better Response to Multikinase Inhibitors in Hepatocellular Carcinoma
Ji Won Han, Ji Hoon Kim, Dong Hyun Kim, Jeong Won Jang, Si Hyun Bae, Jong Young Choi, Seung Kew Yoon, Jaegyoon Ahn, Hyun Yang, Pil Soo Sung
Diagnostics.2023; 13(8): 1453. CrossRef - Immune checkpoint inhibitors in HCC: Cellular, molecular and systemic data
Uasim Harkus, Miriam Wankell, Pranavan Palamuthusingam, Craig McFarlane, Lionel Hebbard
Seminars in Cancer Biology.2022; 86: 799. CrossRef - Crosstalk between tumor-associated macrophages and neighboring cells in hepatocellular carcinoma
Pil Soo Sung
Clinical and Molecular Hepatology.2022; 28(3): 333. CrossRef
- Higher Number of Tumor-Infiltrating PD-L1+ Cells Is Related to Better Response to Multikinase Inhibitors in Hepatocellular Carcinoma


 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION

 First
First Prev
Prev



 Follow JLC on Twitter
Follow JLC on Twitter