Articles
- Page Path
- HOME > J Liver Cancer > Volume 21(2); 2021 > Article
-
Original Article
Transarterial chemoembolization using drug-eluting bead compared with radiofrequency ablation for treatment of single small hepatocellular carcinoma: a pilot non-randomized trial -
Tae Hoon Kim1
 , Na Hye Kim2
, Na Hye Kim2 , Jin Dong Kim1
, Jin Dong Kim1 , Young Nam Kim1
, Young Nam Kim1 , Yu Jin Kim1
, Yu Jin Kim1 , Eun Jung Kim1
, Eun Jung Kim1 , Ki Deok Yoo1
, Ki Deok Yoo1 , Choong Heon Ryu1
, Choong Heon Ryu1 , Ha Hun Song3
, Ha Hun Song3 , Hyun Kim3
, Hyun Kim3
-
Journal of Liver Cancer 2021;21(2):146-154.
DOI: https://doi.org/10.17998/jlc.2021.05.20
Published online: August 11, 2021
1Department of Internal Medicine, Cheju Halla General Hospital, Jeju, Korea
2Yonsei University College of Medicine, Seoul, Korea
3Department of Radiology, Cheju Halla General Hospital, Jeju, Korea
-
Corresponding author: Jin Dong Kim, Department of Internal Medicine, Cheju Halla General Hospital, 65 Doryeong-ro, Jeju 63127, Korea,
Tel. +82-64-740-5082; Fax. +82-64-740-5652, E-mail: motet76@gmail.com - *These authors are co-first authors who contributed equally to this work.
Copyright © 2021 by The Korean Liver Cancer Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 4,925 Views
- 141 Downloads
- 3 Citations
Abstract
-
Background/Aims
- Surgical resection, transplantation, and radiofrequency ablation (RFA) are generally accepted as amenable treatments for small hepatocellular carcinoma (HCC). Recently drug-eluting beads (DEB) which had several treatment advantages were introduced for transarterial chemoembolization (TACE). The aim of this study was to evaluate feasibility and safety of DEB-TACE compared with RFA for the treatment of single small HCC.
-
Methods
- In this pilot non-randomized trial, we assessed retrospective data of 40 patients who underwent DEB-TACE (n=21) or RFA (n=19) for single small (≤3 centimeter in greatest dimension) HCC. The primary outcomes were tumor response and time to recurrence. The secondary outcome was treatment-related complications.
-
Results
- Complete response rate to DEB-TACE and RFA after first follow-up assessment was 90.5% and 94.7%, respectively (P=1.000). During mean follow-up of 87.6 months (95% confidence interval, 74.4–102), 7 patients experienced local recurrence. The 6- and 12-month cumulative local recurrence rate was 5.0% and 21.8% in DEB-TACE vs. 11.1% and 17.0% in RFA group (P=0.877). A total 14 distant intrahepatic recurrences were developed and 12- and 24-month cumulative distant intrahepatic recurrence rate was 20.6% and 42.7% in DEB-TACE vs. 17.2% and 36.3% in RFA group (P=0.844). Two patients experienced gangrenous cholecystitis after DEB-TACE requiring cholecystectomy as treatment-related adverse event.
-
Conclusions
- Tumor response and recurrence rate after single session of DEB-TACE or RFA were similar. DEB-TACE could be applied selectively in patients with a single small HCC if the other therapeutic modality is unfeasible.
- Among primary liver malignancies, hepatocellular carcinoma (HCC) represents the major histological subtype accounting for 70% to 85% of the total liver cancer burden worldwide.1 Although the mainstays of therapy for small HCC are surgical resection and liver transplantation, many HCC patients are not eligible for surgery because of underlying liver dysfunction or organ shortage. Other accepted interventional treatments including radiofrequency ablation (RFA) and percutaneous ethanol injection have been used to treat small HCC based on institutional preference, but a technical difficulty of local treatments may be encountered particularly when tumor is centrally located or adjacent to large vessels, gallbladder or intestine.
- Transarterial chemoembolization (TACE) is commonly used for unresectable or multifocal HCCs that are not amenable to other local treatments.2,3 While embolization induces tumor ischemia by disrupting the blood supply to the tumor, its combination with chemotherapeutic agents results in a strong cytotoxic effect.4,5 Recently, drug-eluting beads (DEB) which had the ability to sequester chemotherapeutic agent from solution and release it in a sustained and controlled mode were introduced for TACE.6 The purpose of this study was to evaluate feasibility and safety of DEB-TACE compared with RFA for the treatment of a single small HCC.
INTRODUCTION
- 1. Ethics statement
- The study protocol conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board (IRB no. 2020-L09-01). The requirement for informed consent from patients was waived due to the retrospective nature of this study.
- 2. Patients
- This is a pilot non-randomized trial through a retrospective chart review in a single center. Consecutive patients who underwent at least one session of DEB-TACE or RFA for first treatment of single small HCC from October 2010 to March 2017 and who met the following eligibility criteria were included. Eligibility criteria were as follows: 1) newly diagnosed, treatment-naïve HCC, 2) Child-Pugh class A or B, 3) Eastern Cooperative Oncology Group (ECOG) performance status 0,1 or 2, and 4) absence of main portal vein thrombosis and extrahepatic metastases.7 Patients at high risk for surgery or those who were unwilling to receive operation in spite of the clinician’s advice were included. We excluded patients with previous histories of intra- or extrahepatic malignancies (n=3), poor hepatic reservoir function of Child-Pugh class C (n=11), medically uncontrolled or recurrent hepatic encephalopathy (n=4), and poor performance status of ECOG 3 or higher (n=7). As a result, a total of 40 patients were finally included in this study. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) reporting guidelines were followed (Supplementary Table 1).
- 3. Diagnosis of HCC
- Diagnosis of a small (≤3 cm in greatest dimension) solitary HCC was made based upon the typical radiological findings (arterial hypervascularity and venous/late phase washout) on dynamic computed tomography (CT). In all patients, a radiological diagnosis of HCC was ascertained by gadoxetic acid (Primovist®, Bayer HealthCare, Leverkusen, Germany)-enhanced magnetic resonance imaging (MRI) following dynamic CT.8,9 On gadoxetic acid-enhanced MRI, lesions satisfying at least two findings within the following criteria were considered as HCC nodules: 1) arterial enhancement with or without portal/equilibrium phase washout, 2) hypointensity on hepatobiliary phase, 3) hyperintensity on T2-weighted image, and 4) hyperintensity on diffusion-weighted image.10
- 4. TACE protocol
- One radiologist with 25 years of experience in intervention performed all procedures. After hepatic and superior mesenteric artery angiography to check for arteriovenous shunts, and identification of arterial tumor supply, feeding arteries were selectively catheterized using a microcatheter. After positioning the microcatheter tip close to the tumor-feeding branch, a mixture of 1 vial of DEB (70–150 or 100–300 μm diameter; DC Bead®, Biocompatibles, Farnham, UK) loaded with doxorubicin (50 or 75 mg) and contrast agent was infused until the flow through the tumor-feeding artery was slowed down.
- 5. RFA protocol
- Under conscious sedation and local anesthesia, all RFA procedures were performed percutaneously by the aforementioned radiologist. LeVeen needle electrode® (Boston Scientific, Marlborough, MA, USA) or Well-Point RF electrode® (STARmed, Goyang, Korea) were used according to the manufacturers’ recommended protocols. Real-time sonographic guidance and monitoring was made for adequate ablation during procedure. In case of invisible HCC at sonographic targeting, CT was applied for adequate needle positioning prior to and post-ablation.
- 6. Radiological follow-up and treatment response
- Primary outcomes were tumor response and local and distant intrahepatic recurrences of HCC. Local recurrence of HCC was defined when enhancing tumor reemerged at the target lesion and distant intrahepatic recurrence was defined when the new HCC was developed at a separate intrahepatic site.11 These were evaluated by regular follow-up with CT scan and/or MRI at 1, 3, and 5–6 months after receiving DEB-TACE or RFA, using the modified Response Evaluation Criteria in Solid Tumors.12 Complete response was defined as disappearance of intratumoral enhancement; partial response was defined as a ≥30% decrease in the sum of the diameters of viable lesion; progressive disease was defined as an increase of ≥20% in the sum of diameters of viable tumor; and stable disease was defined as any cases that did not qualify as either partial response of progressive disease. Patients who were diagnosed with HCC recurrence after initial treatment were assigned to sequential therapeutic modalities.
- 7. Safety
- The secondary endpoint was treatment-related complication. Laboratory tests were performed 1, 3–7, 14 days and every 4–12 weeks after treatment. Adverse events were assessed using the Common Terminology Criteria for Adverse Events (version 4.0).13
- 8. Statistical analysis
- Results are summarized either as number (%), median (ranges) or mean (95% confidence interval [CI]) as appropriate. Continuous data were analyzed using the Mann-Whitney U test or student’s t-test. Categorical data were evaluated using the Fisher exact test or chi-square test. Insufficient response to first treatment was censored at the time of first evaluation of therapeutic response. Follow-up loss or death without tumor recurrence was censored at the time of the last follow-up. Recurrence rates and curves were obtained using the Kaplan-Meier method and compared using the log-rank test. Multivariate Cox regression was performed to identify independent factors associated with tumor recurrence using variables with P-values less than 0.1 in univariate analysis. A P-value of less than 0.05 (2-tailed) was considered statistically significant. Statistical analyses were performed using MedCalc for Windows, version 19.4 (MedCalc Software, Ostend, Belgium).
METHODS
- 1. Clinical characteristics of the patients
- The baseline characteristics of the patients are listed in Table 1. Twenty-one patients (52.5%) underwent DEB-TACE and 19 patients underwent RFA (11 ultrasound-guided and 8 CT-guided). The patients included 27 men and 13 women with median age of 67.5 years (range, 43–84). About 87% of the patients had Child-Pugh grade A liver function, and 60% of patients had ECOG performance grade of 0 while the remaining were either grade 1 or 2. The median tumor diameter was 2.0 cm (range, 1.0–3.0). There was no difference between the groups with regard to tumor stage (P =0.936). Although Child-Pugh scores of the two groups were not significantly different, the serum albumin of the RFA group was lower than that of the DEB-TACE group (4.2 vs. 3.5 g/dL, P =0.028) and prothrombin time international normalized ratio of the RFA group was higher than that of DEB-TACE groups (1.10 vs. 1.20, P =0.011).
- 2. Tumor response
- The target tumor responses evaluated after first treatment are summarized in Table 2. Complete response rates in DEB-TACE and RFA groups were 90.5% and 94.7%, respectively (P =1.000). In DEB-TACE group, partial response and stable disease rates were equally 4.8%. One patient (5.3%) in the RFA group showed progressive disease.
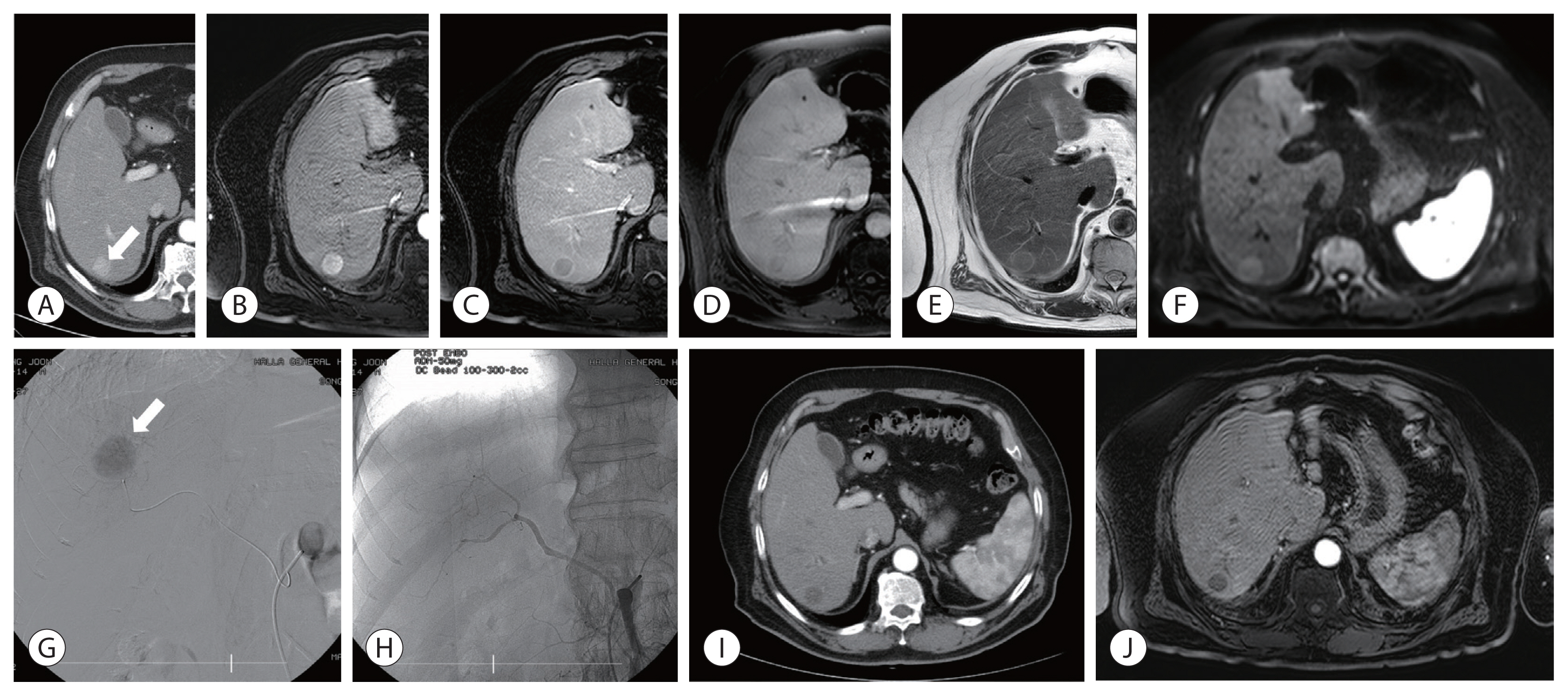
- Fig. 1. describes the case of a patient with 2.0 cm-sized solitary enhancing nodule who experienced a complete necrosis on follow-up CT after a single session of DEB-TACE and underlines the absence of local tumor recurrence for 28.6 months.
- 3. Local and distant recurrences and risk factors related to recurrences
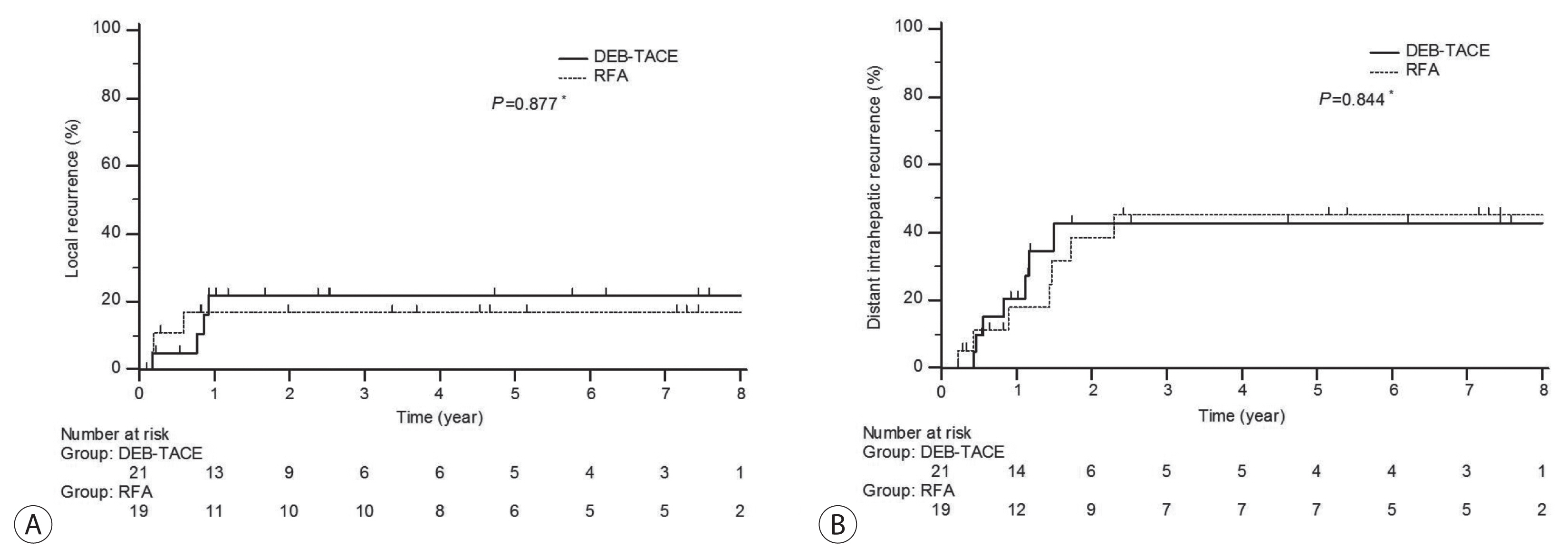
- Overall mean follow-up duration for local recurrence was 87.6 months (95% CI, 74.4–102) and 65.6 months (95% CI, 48.6–82.6) for distant intrahepatic recurrence. Of the 21 patients who underwent DEB-TACE, one patient had partial response and one patient showed stable disease on first follow-up imaging. Local recurrences were noted in another four patients. Among 19 patients who received RFA, one patient showed progressive disease at first response evaluation and another three patients developed local recurrences. The 6- and 12-month cumulative local recurrence rate was 5.0% and 21.8% in DEB-TACE vs. 11.1% and 17.0% in RFA group (Fig. 2A, P =0.877) and reached a plateau in 12 months. Distant intrahepatic recurrences developed in 14 cases during follow-up (7 and 7, respective groups). The 12- and 24-month cumulative distant intrahepatic recurrence rate was 20.6% and 42.7% in DEB-TACE vs. 17.2% and 36.3% in RFA group (Fig. 2B, P=0.844).
- On Cox regression analysis with multivariable adjustment, there was no associated factor regarding local recurrence. Distant intrahepatic recurrence was significantly associated with albumin level and tumor size (Table 3). Treatment modality was not an independent risk factor for local or distant intrahepatic recurrences.
- 4. Safety
- As treatment-related adverse events, there were no grade 3–4 laboratory toxicities in either group within 4 weeks and no mortality directly related to the procedures. Development of post-embolization or post-ablation syndrome under control with analgesic or antipyretic drugs was shown in 6 patients (28.6%) in DEB-TACE and 4 patients (21.1%) among RFA group. In RFA group, two patients experienced small pneumothorax (<2 cm rim present between the lung edge and chest wall) which was absorbed with supplemental oxygen within 1–2 days.
- Two patients (9.5%) experienced gangrenous cholecystitis requiring cholecystectomy in DEB-TACE group. Fig. 3. illustrates one of these patients who underwent emergent operation and the biopsy resulted in ischemia due to deposition of embolic particles in the vessel. Another patient experienced liver abscess which required percutaneous catheter drainage.
RESULTS
- In this study, we assessed retrospective data of 40 patients who underwent DEB-TACE or RFA for initial treatment of single small HCC. During a mean follow-up period of 87.6 months (95% CI, 74.4–102), 7 patients experienced local recurrence and 14 patients experienced distant intra-hepatic recurrence. The 6- and 12-month cumulative local recurrence rate was 5.0% and 21.8% in DEB-TACE vs. 11.1% and 17.0% in RFA group (P =0.877). The 12- and 24-month cumulative distant intrahepatic recurrence rate was 20.6% and 42.7% in DEB-TACE vs. 17.2% and 36.3% in RFA group (P =0.844).
- In several practice guidelines, surgical resection and RFA are generally regarded as therapeutic strategies for patients of small HCC who have relatively good liver function and performance status.9,14–16 Previous articles regarding outcomes of RFA for treatment of small HCC (<5 cm) showed complete ablation in over 95% of cases.17 Two studies analyzed effectiveness of RFA for single small HCC up to 3 cm and local recurrences were reported 23.5–28.5% at 3 years and 27.9–32.1% at 5 years.18,19 However, there are several technical limitations and RFA-related complications although RFA appears to provide reasonable results comparable to surgical resection. Diminished effect or technical difficulty of RFA could be encountered when the tumor is located adjacent to a major vessel (so-called “heat sink effect”), biliary tracts, critical extrahepatic organs (stomach, small and large intestines, gallbladder) or other structures (diaphragm, abdominal wall).17
- From the anatomical aspect, the endoarterial approach could afford additionally safe therapeutic modality for aforementioned ‘difficult-to-ablate’ HCC cases. Compared with conventional TACE, DEB-TACE has been recognized to minimize drug-related side effects because it significantly improves drug delivery system by maximizing a constant concentration of the chemotherapeutic agents transported to the tumor while reducing the amount reaching the systemic circulation. 20,21 In previous retrospective comparative study of conventional vs. DEB-TACE for small HCC, DEB-TACE provided more procedural safety than conventional TACE and tumor responses between two groups were not significantly different.22 However, as the authors revealed as a study limitation, the study focused on early-term data at 1 year.
- Therefore, it is needed for subsequent studies on DEB-TACE compared to RFA, which is already known to be an acceptable curative treatment for small HCC. In this study, we analyzed relatively long-term follow-up data of patients who underwent DEB-TACE or RFA, and there were no significant differences in tumor response, local and distant intrahepatic recurrences between two groups. Two cases of cholecystitis as a complication of DEB-TACE developed early after initiation of DEB-TACE in our center and no additional non-target embolic side effects were reported subsequently.
- The following limitations should be noted: 1) The study was a retrospective, single-center study and the number of enrolled patients was limited; and 2) the efficacy of DEB-TACE could be underestimated because treatment response was evaluated after one session of DEB-TACE or RFA. In a previous prospective DEB-TACE study, patients received up to three sessions of chemoembolization at 2-month intervals. 23 Further multicenter retrospective or prospective studies are needed to compare therapeutic efficacy between DEB-TACE and RFA or surgical resection.
- In conclusion, tumor response and recurrence after DEB-TACE and RFA for treatment of small HCC is not significantly different in this pilot study. Considering the procedure-related complications, DEB-TACE could be a bridging therapeutic option for local disease control in selective patients of small HCC if other therapeutic modalities are unfeasible.
DISCUSSION
-
Conflicts of Interest
The authors have no conflicts of interest to disclose.
-
Ethics Statement
This study protocol conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board of Cheju Halla General Hospital (IRB no. 2020-L09-01). The requirement for informed consent from patients was waived due to the retrospective nature of this study.
-
Funding Statement
No funding to declare.
-
Data Availability
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
-
Author Contribution
Study concept and design: JDK.
Data acquisition: THK, NHK, YNK, EJK, KDY, HHS, HK.
Data analysis and interpretation: JDK, THK, NHK, YJK.
Drafting of the manuscript: THK, NHK.
Critical revision of the manuscript for important intellectual content: YJK, CHR.
Statistical analysis: THK, NHK, JDK.
Study supervision: JDK.
All authors have reviewed and approved the final version of manuscript.
Article information
Supplementary Material

| Variable | DEB-TACE group (n=21) | RFA group (n=19) | P-value* |
|---|---|---|---|
| Age (years) | 65 (43–84) | 69 (47–76) | 0.978 |
| Sex (male) | 12 (57.1) | 15 (78.9) | 0.186 |
| ECOG performance status (grade 0/1/2) | 13/5/3 (61.9/23.8/14.3) | 11/6/2 (57.9/31.6/10.5) | 0.991 |
| Etiology (HBV/HCV/alcohol/other) | 15/3/2/1 (71.4/14.3/9.5/4.8) | 7/5/6/1 (36.8/26.3/31.6/5.3) | 0.053 |
| Child-Pugh score (5/6/7) | 17/2/2 (81.0/9.5/9.5) | 13/3/3 (68.4/15.8/15.8) | 0.393 |
| Tumor size (cm) | 2.0 (1.0–3.0) | 1.9 (1.0–3.0) | 0.595 |
| mUICC stage (I/II) | 13/8 (61.9/38.1) | 12/7 (63.2/36.8) | 0.936 |
| WBC (/mm3) | 4,520 (1,800–9,200) | 5,310 (2,970–10,370) | 0.542 |
| Hemoglobin (g/dL) | 13.4 (10.5–17.9) | 11.9 (9.1–16.0) | 0.172 |
| Platelet counts (×1,000/mm3) | 138 (47–287) | 89 (34–244) | 0.063 |
| Albumin (g/dL) | 4.2 (3.3–4.9) | 3.5 (3.1–4.9) | 0.028 |
| Bilirubin (mg/dL) | 0.81 (0.41–1.49) | 1.0 (0.23–4.0) | 0.136 |
| Prothrombin time (INR) | 1.10 (0.90–1.23) | 1.20 (0.95–1.80) | 0.011 |
| AFP (ng/mL) | 8.23 (1.65–3,340) | 14.01 (1.52–5,845) | 0.278 |
| PIVKA-II (mAU/mL) | 33 (12–343) | 68.5 (12–2,745) | 0.075 |
Values are presented as number (%) or median (range).
DEB-TACE, drug-eluting bead transarterial chemoembolization; RFA, radiofrequency ablation; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; mUICC, modified International Union Against Cancer; WBC, white blood cell; INR, international normalized ratio; AFP, α-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist-II.
* P-values were calculated using the Mann-Whitney U test, Student’s t-test, Fisher exact test or chi-square test.
| Tumor response | Complete response | Partial response | Stable disease | Progressive disease |
|---|---|---|---|---|
| DEB-TACE | 19 (90.5) | 1 (4.8) | 1 (4.8) | 0 |
| RFA | 18 (94.7) | 0 | 0 | 1 (5.3) |
| P-value* | 1.000 | 1.000 | 1.000 | 0.475 |
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P-value* | HR (95% CI) | P-value* | |
| Local recurrence | ||||
| PIVKA | 1.001 (0.999–1.002) | 0.086 | - | - |
| Treatment modality | 0.886 (0.199–3.975) | 0.877 | - | - |
|
|
||||
| Distant intrahepatic recurrence | ||||
| Albumin | 0.410 (0.169–0.993) | 0.048 | 0.379 (0.153–0.937) | 0.036 |
| mUICC | 2.920 (1.007–8.467) | 0.049 | 0.499 (0.083–3.010) | 0.449 |
| Tumor size | 3.651 (1.347–9.899) | 0.011 | 8.197 (1.110–60.539) | 0.039 |
| Treatment modality | 0.957 (0.333–2.752) | 0.935 | - | - |
- 1. Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006;45:529−538.ArticlePubMed
- 2. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005;42:1208−1236.ArticlePubMed
- 3. Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008;100:698−711.ArticlePubMed
- 4. Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology 2004;127(5 Suppl 1): S179−S188.ArticlePubMed
- 5. Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology 2010;52:762−773.ArticlePubMed
- 6. Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res 2006;12:2563−2567.ArticlePubMed
- 7. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649−655.ArticlePubMed
- 8. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358−380.ArticlePubMed
- 9. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182−236.ArticlePubMed
- 10. Ahn SS, Kim MJ, Lim JS, Hong HS, Chung YE, Choi JY. Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology 2010;255:459−466.ArticlePubMed
- 11. Doyle A, Gorgen A, Muaddi H, Aravinthan AD, Issachar A, Mironov O, et al. Outcomes of radiofrequency ablation as first-line therapy for hepatocellular carcinoma less than 3 cm in potentially transplantable patients. J Hepatol 2019;70:866−873.ArticlePubMed
- 12. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52−60.ArticlePubMed
- 13. National Cancer Institute. Common terminology criteria for adverse events (CTCAE) version 4.0 2009;[Internet]. Bethesda (MD): National Cancer Institute; [cited 2020 Nov 14]. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CT-CAE_4.03_2010-06-14_QuickReference_5x7.pdf
- 14. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723−750.ArticlePubMed
- 15. Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317−370.ArticlePubMed
- 16. Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver 2019;13:227−299.ArticlePubMedPMC
- 17. Nault JC, Sutter O, Nahon P, Ganne-Carrié N, Séror O. Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J Hepatol 2018;68:783−797.ArticlePubMed
- 18. Brunello F, Cantamessa A, Gaia S, Carucci P, Rolle E, Castiglione A, et al. Radiofrequency ablation: technical and clinical long-term outcomes for single hepatocellular carcinoma up to 30 mm. Eur J Gastroenterol Hepatol 2013;25:842−849.PubMed
- 19. Francica G, Saviano A, De Sio I, De Matthaeis N, Brunello F, Cantamessa A, et al. Long-term effectiveness of radiofrequency ablation for solitary small hepatocellular carcinoma: a retrospective analysis of 363 patients. Dig Liver Dis 2013;45:336−341.ArticlePubMed
- 20. Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 2007;46:474−481.ArticlePubMed
- 21. Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol 2007;5:1100−1108.ArticlePubMed
- 22. Lee KH, Joo SM, Yum TJ, Jung SH. Conventional versus drug-eluting beads trans-arterial chemoembolization for treatment of hepatocellular carcinoma at very early and early stages. J Liver Cancer 2017;17:144−152.Article
- 23. Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41−52.ArticlePubMed
References
Figure & Data
References
Citations

- Incidence and Risk Factors of Acute Ischemic Cholecystitis after Transarterial Chemoembolization: Correlation with Cone Beam CT Findings
Jong Yeong Kim, Jung Suk Oh, Ho Jong Chun, Su Ho Kim
Journal of the Korean Society of Radiology.2024; 85(2): 363. CrossRef - Drug-Eluting Bead Transarterial Chemoembolization Versus Radiofrequency Ablation as an Initial Treatment of Single Small (≤ 3 cm) Hepatocellular Carcinoma
Somin Lee, Yong Yeon Jeong, Byung Chan Lee, Sang Soo Shin, Suk Hee Heo, Hyoung Ook Kim, Chan Park, Won Gi Jeong
Journal of Korean Medical Science.2023;[Epub] CrossRef - Comparable Outcomes in Early Hepatocellular Carcinomas Treated with Trans-Arterial Chemoembolization and Radiofrequency Ablation
Benjamin Wei Rong Tay, Daniel Q. Huang, Muthiah Mark, Neo Wee Thong, Lee Guan Huei, Lim Seng Gee, Low How Cheng, Lee Yin Mei, Prem Thurairajah, Lim Jia Chen, Cheng Han Ng, Wen Hui Lim, Darren Jun Hao Tan, Da Costa Maureen, Kow Wei Chieh Alfred, Iyer Shrid
Biomedicines.2022; 10(10): 2361. CrossRef

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION
 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation

 Follow JLC on Twitter
Follow JLC on Twitter