Search
- Page Path
- HOME > Search
Original Articles
- Effect of direct-acting antivirals for hepatitis C virus-related hepatocellular carcinoma recurrence and death after curative treatment
- Young-Hwan Ahn, Heirim Lee, Ji Eun Han, Hyo Jung Cho, Jae Youn Cheong, Bumhee Park, Soon Sun Kim
- J Liver Cancer. 2022;22(2):125-135. Published online June 28, 2022
- DOI: https://doi.org/10.17998/jlc.2022.05.24
- 2,971 Views
- 78 Downloads
- 4 Citations
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background/Aim
There has been a long-standing debate about the association of directacting antiviral (DAA) therapy and hepatocellular carcinoma (HCC) recurrence. This study aimed to investigate the association between DAA therapy and HCC recurrence after curative therapy.
Methods
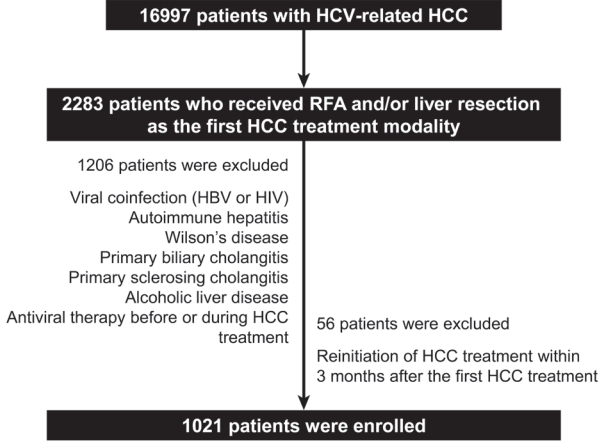
We retrospectively enrolled 1,021 patients with HCV-related (hepatitis C virus) HCC who underwent radiofrequency ablation (RFA), liver resection, or both as the first treatment modality from January 2007 to December 2016 and without a history of HCV therapy before HCC treatment from a nationwide database. The effect of HCV treatment on HCC recurrence and all-cause mortality was also investigated.
Results
Among the 1,021 patients, 77 (7.5%) were treated with DAA, 14 (1.4%) were treated with interferon-based therapy, and 930 (91.1%) did not receive HCV therapy. DAA therapy was an independent prognostic factor for lower HCC recurrence rate (hazard ratio [HR], 0.04; 95% confidence interval [CI], 0.006-0.289; P=0.001 for landmarks at 6 months after HCC treatment and HR, 0.05; 95% CI, 0.007-0.354; P=0.003 for landmarks at 1 year). Furthermore, DAA therapy was associated with lower all-cause mortality (HR, 0.049; 95% CI, 0.007-0.349; P=0.003 for landmarks at 6 months and HR, 0.063; 95% CI, 0.009-0.451; P=0.006 for landmarks at 1 year).
Conclusions
DAA therapy after curative HCC treatment can decrease HCC recurrence and all-cause mortality compared to interferon-based therapy or no antiviral therapy. Therefore, clinicians should consider administering DAA therapy after curative HCC treatment in patients with HCV-related HCC. -
Citations
Citations to this article as recorded by- Comparison of Surgical Resection and Radiofrequency Ablation in Elderly Patients with Hepatocellular Carcinoma
Jun Il Kim, Jayoun Lee, Gi Hong Choi, Min Woo Lee, Dong Ah Park, Jeong-Ju Yoo
Digestive Diseases and Sciences.2024; 69(3): 1055. CrossRef - Analyzing risk factors and developing a stratification system for hepatocellular carcinoma recurrence after interferon-free direct-acting antiviral therapy in chronic hepatitis C patients
Chih-Hsuan Luan, Pin-Shuo Su, Chi-Jen Chu, Chung-Chi Lin, Chien-Wei Su, Jiing-Chyuan Luo, I-Cheng Lee, Chen-Ta Chi, Shou-Dong Lee, Yuan-Jen Wang, Fa-Yauh Lee, Yi-Hsiang Huang, Ming-Chih Hou
Journal of the Chinese Medical Association.2024; 87(4): 357. CrossRef - Addition of Kidney Dysfunction Type to MELD-Na for the Prediction of Survival in Cirrhotic Patients Awaiting Liver Transplantation in Comparison with MELD 3.0 with Albumin
Kyeong-Min Yeom, Jong-In Chang, Jeong-Ju Yoo, Ji Eun Moon, Dong Hyun Sinn, Young Seok Kim, Sang Gyune Kim
Diagnostics.2023; 14(1): 39. CrossRef - Is direct-acting antiviral treatment beneficial or harmful for patients with hepatitis C virus-related hepatocellular carcinoma?
Hye Won Lee
Journal of Liver Cancer.2022; 22(2): 91. CrossRef
- Comparison of Surgical Resection and Radiofrequency Ablation in Elderly Patients with Hepatocellular Carcinoma

- Tenofovir and Entecavir Have Similar Renal Adverse Events on Hepatocellular Carcinoma Patients Treated with Transarterial Chemoembolization
- Young Youn Cho, Young Hwan Choi, Su Jong Yu, Eun Ju Cho, Jeong-Hoon Lee, Yoon Jun Kim, Jung-Hwan Yoon
- J Liver Cancer. 2019;19(2):128-135. Published online September 30, 2019
- DOI: https://doi.org/10.17998/jlc.19.2.128
- 3,813 Views
- 47 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
PDF - Background/Aim
s: Tenofovir disoproxil fumarate (TDF) is potentially nephrotoxic in chronic hepatitis B patients. Hepatocellular carcinoma (HCC) patients treated using transarterial chemoembolization (TACE) are at an increased risk of renal injury. The aim of this study was to determine whether TDF is associated with more renal adverse events than entecavir (ETV) in HCC patients treated with TACE.
Methods
In this retrospective single-center study, we selected 53 HCC patients who were treated with TDF from January 2012 to July 2013 and had their first TACE procedure in the same period. These patients were matched by age and sex to patients treated with ETV.
Results
There were no significant differences in baseline characteristics, including HCC factors, and nephrotoxic drug use, between the two groups. The median follow-up period was 17.0 and 20.0 months for the TDF and ETV groups, respectively. There was no difference during the follow-up period between the TDF and ETV groups in the increase in creatinine over 0.5 mg/dL (17.0% and 17.0%, P=1.00, respectively) and the decrease in eGFR over 25% (43.4% and 41.5%, P=0.84, respectively). Multivariate analysis revealed that Child-Pugh class over B (hazard ratio [HR], 7.30; 95% confidence interval [CI] 2.79-19.10; P<0.01) was associated with increase in creatinine, and Child-Pugh class over B (HR, 82.74; 95% CI 12.31-555.83; P<0.01) and Barcelona-Clinic Liver Cancer stage over B (HR, 14.93; 95% CI 1.60-139.51; P=0.02) were associated with decrease in eGFR.
Conclusions
TDF has comparable safety to that of ETV for HCC patients undergoing TACE. -
Citations
Citations to this article as recorded by- Big Data Information under Proportional Hazard Mathematical Model in Analysis of Hepatitis B Virus Infection Data of Patients with Interventional Liver Cancer through Antiviral Therapy of Entecavir
Yichi Zhang, Shuai Zhao, Han Ding, Xiaoling Song, Huijie Miao, Xuya Cui, Jian Wang, Bing Han, Enas Abdulhay
Journal of Healthcare Engineering.2021; 2021: 1. CrossRef
- Big Data Information under Proportional Hazard Mathematical Model in Analysis of Hepatitis B Virus Infection Data of Patients with Interventional Liver Cancer through Antiviral Therapy of Entecavir

- Survival Benefit of Antiviral Agents for Hepatocellular Carcinoma Patients Treated with Sorafenib
- Jeong Han Kim, Hyung Min Yu, Yong Hwang, Soon Young Ko, Won Hyeok Choe, So Young Kwon
- J Liver Cancer. 2016;16(1):23-30. Published online March 31, 2016
- DOI: https://doi.org/10.17998/jlc.16.1.23
- 1,078 Views
- 12 Downloads
-
 Abstract
Abstract
 PDF
PDF - Background/Aim
s: Nucleos(t)ide analogues (NAs) help reduce the recurrence rate after the curative treatment of hepatitis B related hepatocellular carcinoma (HCC). Sorafenib has been shown to improve survival of advanced HCC patients. Whether antiviral therapy with NAs could help such patients is unknown. Our aim is to investigate the usefulness of antiviral therapy for advanced-stage HCC treated with sorafenib.
Methods
We performed a retrospective cohort study in advanced-stage HCC patients treated with sorafenib between June 2007 and December 2013. Patients in group A (the nonantiviral therapy group) were treated with sorafenib alone. Those in group B (the antiviral therapy group) were treated with sorafenib and NAs. Progression-free survival (PS) and overall survival (OS) were compared between these two groups.
Results
Finally, 23 patients in group A and 40 patients in group B were enrolled in the study. The mean number of days of treatment with sorafenib was 79 (34-231) days and 96 (33-449) days for group A and B, respectively (P=0.286). The mean PS of group A and B was 97 (14-449) days and 51 (0-461) days, respectively (P=0.068). The OS was 154 (44-741) days in group A and 138 (30-1,025) days in group B (P=0.665). PS and OS showed no significant difference between the two groups.
Conclusions
This study shows that there was no significant survival gain of using antiviral therapy in patients with advanced-stage HCC treated with sorafenib. In consideration of costeffectiveness, antiviral therapy may be not mandatory. (J Liver Cancer 2016;16:23-30)


 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION

 First
First Prev
Prev



 Follow JLC on Twitter
Follow JLC on Twitter