Articles
- Page Path
- HOME > J Liver Cancer > Volume 23(1); 2023 > Article
-
Original Article
Current status of ultrasonography in national cancer surveillance program for hepatocellular carcinoma in South Korea: a large-scale multicenter study -
Sun Hong Yoo1*
 , Soon Sun Kim2*
, Soon Sun Kim2* , Sang Gyune Kim3
, Sang Gyune Kim3 , Jung Hyun Kwon1
, Jung Hyun Kwon1 , Han-Ah Lee4
, Han-Ah Lee4 , Yeon Seok Seo5
, Yeon Seok Seo5 , Young Kul Jung6
, Young Kul Jung6 , Hyung Joon Yim6
, Hyung Joon Yim6 , Do Seon Song7
, Do Seon Song7 , Seong Hee Kang6
, Seong Hee Kang6 , Moon Young Kim8
, Moon Young Kim8 , Young-Hwan Ahn2
, Young-Hwan Ahn2 , Jieun Han2
, Jieun Han2 , Young Seok Kim3
, Young Seok Kim3 , Young Chang9
, Young Chang9 , Soung Won Jeong9
, Soung Won Jeong9 , Jae Young Jang9
, Jae Young Jang9 , Jeong-Ju Yoo3
, Jeong-Ju Yoo3
-
Journal of Liver Cancer 2023;23(1):189-201.
DOI: https://doi.org/10.17998/jlc.2023.03.11
Published online: March 24, 2023
1Department of Internal Medicine, Incheon St. Mary’s Hospital, The Catholic University of Korea, Incheon, Korea
2Department of Internal Medicine, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea
3Department of Gastroenterology and Hepatology, Soonchunhyang University College of Medicine, Bucheon, Korea
4Department of Internal Medicine, Ewha Womans University College of Medicine, Seoul, Korea
5Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
6Department of Internal Medicine, Korea University Ansan Hospital, Ansan, Korea
7Department of Internal Medicine, St. Vincent`s Hospital, The Catholic University of Korea, Suwon, Korea
8Department of Internal Medicine, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea
9Division of Gastroenterology and Hepatology, Department of Internal Medicine, Soonchunhyang University College of Medicine, Seoul, Korea
-
Corresponding author: Jeong-Ju Yoo, Department of Gastroenterology and Hepatology, Liver Clinic, Digestive Research Center, Soonchunhyang University Hospital Bucheon, 170 Jomaru-ro, Bucheon 14584, Korea
Tel. +82-32-621-5215, Fax. +82-32-621-6079 E-mail: puby17@naver.com - *These two authors contributed equally to this work.
© 2023 The Korean Liver Cancer Association.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,004 Views
- 71 Downloads
- 2 Citations
Abstract
-
Background/Aim
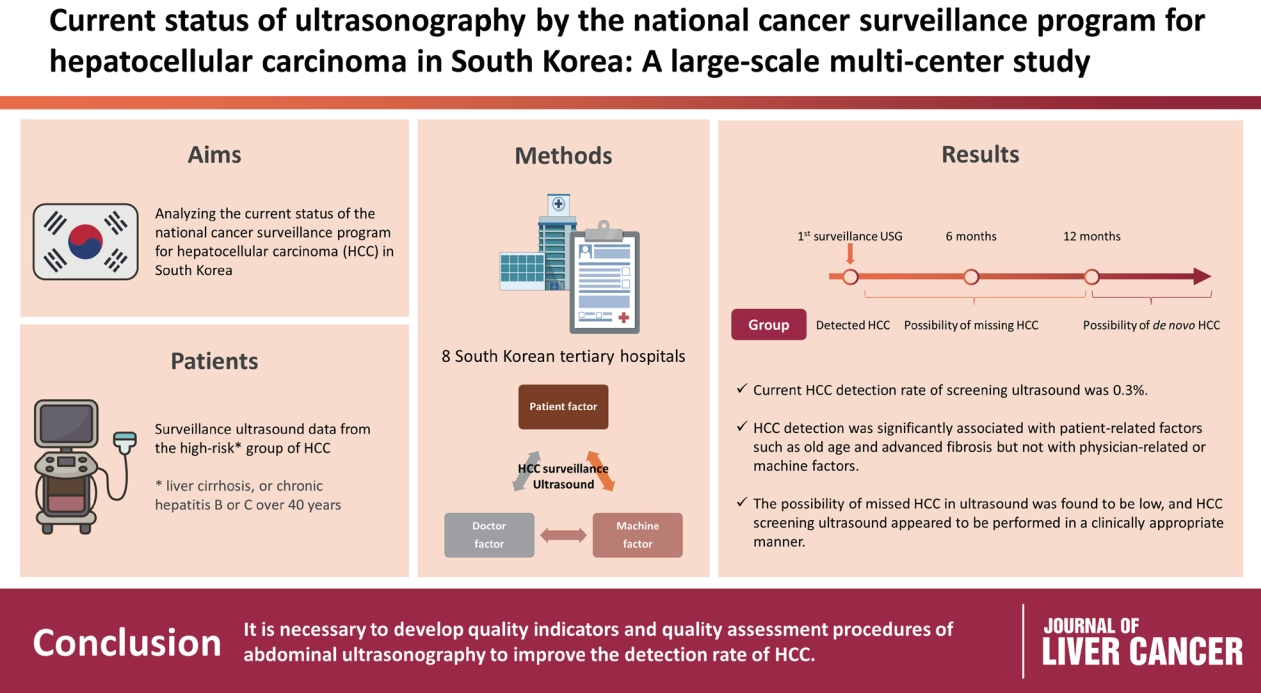
- Abdominal ultrasonography (USG) is recommended as a surveillance test for high-risk groups for hepatocellular carcinoma (HCC). This study aimed to analyze the current status of the national cancer surveillance program for HCC in South Korea and investigate the effects of patient-, physician-, and machine-related factors on HCC detection sensitivity.
-
Methods
- This multicenter retrospective cohort study collected surveillance USG data from the high-risk group for HCC (liver cirrhosis or chronic hepatitis B or C >40 years of age) at eight South Korean tertiary hospitals in 2017.
-
Results
- In 2017, 45 experienced hepatologists or radiologists performed 8,512 USG examinations. The physicians had a mean 15.0±8.3 years of experience; more hepatologists (61.4%) than radiologists (38.6%) participated. Each USG scan took a mean 12.2±3.4 minutes. The HCC detection rate by surveillance USG was 0.3% (n=23). Over 27 months of follow-up, an additional 135 patients (0.7%) developed new HCC. The patients were classified into three groups based on timing of HCC diagnosis since the 1st surveillance USG, and no significant intergroup difference in HCC characteristics was noted. HCC detection was significantly associated with patient-related factors, such as old age and advanced fibrosis, but not with physician- or machine-related factors.
-
Conclusions
- This is the first study of the current status of USG as a surveillance method for HCC at tertiary hospitals in South Korea. It is necessary to develop quality indicators and quality assessment procedures for USG to improve the detection rate of HCC.
- Unlike other cancers, hepatocellular carcinoma (HCC) is frequently found in high-risk groups, for which surveillance tests are recommended.1 Surveillance tests can reduce cancer-related deaths by enabling the detection of HCC at an earlier stage and increasing the chances of cure through interventions.2 The currently most commonly recommended surveillance tests are abdominal ultrasonography (USG) and/or alpha-fetoprotein (AFP) determination.3
- In South Korea, the national cancer screening project is well established, and individuals at a higher risk of HCC undergo USG tests every 6 months.4 Additionally, since 2018, the benefits of upper USG have been made available to the general public, with 80% of the cost covered by the national healthcare system. However, the results of USG tests are more subject to interpretation than those of computed tomography (CT) and magnetic resonance imaging (MRI) and may vary depending on the patient’s condition or examiner’s expertise.5 However, the quality indicators for USG examinations have not yet been established. It is worth noting that several quality indicators for colonoscopy have been developed, including adenoma detection rate, withdrawal time, and bowel preparation quality.6 In the case of USG examinations, there is no known unique quality indicator to assess factors such as appropriate patient selection, examiner experience, or examination duration. The development of quality indicators is crucial for the effective use of USG as a surveillance test for HCC detection. Patient-, physician-, and machine-related factors can play important roles in the USGbased diagnosis of HCC. Physician- and machine-related factors are modifiable and can easily be incorporated as quality indicators.
- The primary purpose of the present study was to analyze the current status of the national cancer surveillance program for HCC and its HCC detection rate. The secondary purpose was to investigate the effects of patient-, physician-, and machine-related factors on HCC detection sensitivity.
INTRODUCTION
- 1. Data source
- In South Korea, the national cancer surveillance program for HCC is available for individuals who have been identified as being at high risk. The high-risk group for HCC was defined as individuals >40 years of age with chronic hepatitis B or C or those with liver cirrhosis regardless of age. For individuals in the high-risk group, regular surveillance tests, consisting of USG and AFP, were performed every 6 months. The present study investigated patients who underwent HCC surveillance tests at eight tertiary institutions in the Seoul and Gyeongin metropolitan areas in South Korea from January 1, 2017 to December 31, 2017. The results of all surveillance tests were included from tests performed at health screening centers located in tertiary hospitals, radiology departments, and gastroenterology departments. Since one purpose of this study was to verify the HCC diagnosis rate through surveillance USG, patients with a history of undergoing a CT or MRI scan within 6 months prior to the USG were excluded because of the possibility of an altered USG sensitivity rate. Patients with a history of HCC were also excluded. The final analyses were based on data obtained from 7,989 subjects.
- The study protocol was approved by the Institutional Review Board (IRB) of each hospital (SCHBC 2019-09-009- 001; date of registration: December 26, 2019) and conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki. Each IRB waived the requirement for informed consent owing to the retrospective nature of the study. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) reporting guidelines were followed (Supplementary Table 1).
- 2. Data collection and classification of HCC
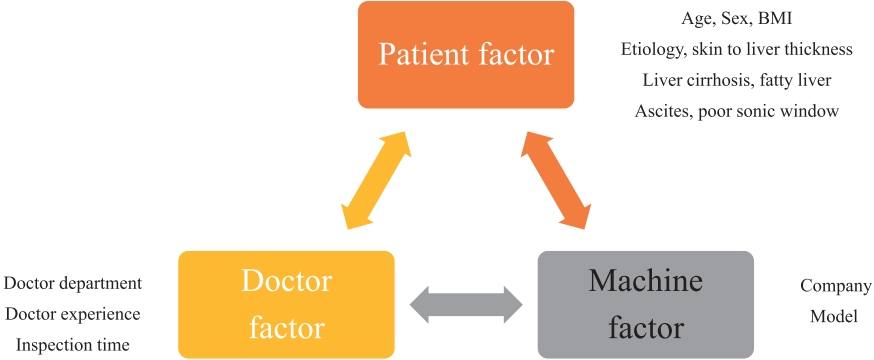
- Three aspects of the surveillance test were investigated: patient-related (age, sex, body mass index, etiology, liver cirrhosis, fatty liver, ascites, poor sonic window, liver function, and other laboratory tests), physician-related (department, experience, duration of the examination), and machine-related (manufacturer, model) factors (Fig. 1). Skin-to-liver thickness was defined as the shortest distance from the skin to the liver capsule of the right lobe in the intercostal view.
- Considering the doubling time for HCC tumor volume, HCC was classified as follows based on the timing of HCC detection since the 1st surveillance USG: 1) detected HCC, HCCs found on the 1st surveillance test; 2) possibility of missing HCC found within 12 months after the 1st surveillance USG; and 3) possibility of de novo HCC found 12 months after the 1st surveillance USG. Fig. 2 shows a brief schematic of this classification. HCC was defined as a case in which suspicious lesions were found through USG and subsequent imaging tests, such as CT or MRI, confirmed the findings according to the Korean Liver Cancer AssociationNational Cancer Center practice guidelines.7
- 3. Statistical analysis
- Frequencies and percentages were used for descriptive statistics. Significant intergroup differences were investigated using the chi-squared test for categorical variables and Student’s t-test for continuous variables. A logistic regression analysis was performed to assess the relationship between in-hospital mortality and other factors. Only the factors significant in the univariate analysis were included in the multivariate analysis. All statistical analyses were performed using R version 4.3.1 (The R Foundation for Statistical Computing; Vienna, Austria). Statistical significance was set at P<0.05.
METHODS
- 1. Baseline characteristics of HCC surveillance subjects
- In 2017, 7,989 patients underwent USG at eight tertiary hospitals for HCC surveillance. The subjects’ baseline characteristics are presented in Table 1. There were 4,531 males (56.7%), and the mean age was 53.6±11.0 years. The most common underlying disease was hepatitis B virus (HBV) (75.0%), followed by hepatitis C virus (HCV) (11.3%), others (9.2%), and alcohol (4.5%). Of the 6,891 patients with viral hepatitis (HBV or HCV), 4,637 (69.7%) received antiviral therapy.
- The average fibrosis-4 (FIB-4) and aminotransferase to platelet ratio index (APRI) scores were 2.2 and 0.6, respectively. Among the participants, 28.8% had cirrhosis. Of these cirrhotic patients, 614 (26.7%) had Child-Pugh class B or C. A total of 93 patients (1.2%) had ascites, while 28 (0.4%) had any grade of hepatic encephalopathy. On the USG screen, the average skin to liver thickness of the patients was 1.8±0.5 cm; 510 subjects (6.4%) had a poor sonic window.
- Regarding physician-related factors, HCC surveillance tests were performed by expert gastroenterologists in 4,919 subjects (61.6%) and expert radiologists in 3,070 subjects (38.4%). The average physician USG experience was 15.1±8.3 years. The mean USG duration was 12.2±3.4 minutes. Regarding machine-related factors, the most commonly used abdominal USG machines were manufactured by TOSHIBA (45.4%), followed by GE Healthcare (39.3%) and PHILIPS (8.6%).
- 2. HCC detection rate through surveillance USG test
- The total number of patients diagnosed with HCC through the 1st USG surveillance test was 23 (0.3% of all subjects). Among the 23 patients (0.3%), HCC was not found on the 1st surveillance USG test but was found within 12 months after the 1st surveillance USG. Additionally, HCC was not found in the 1st surveillance USG test but was found more than 12 months after the 1st surveillance USG test in 112 patients (1.4%). In summary, the HCC diagnosis rate in the 1st surveillance USG test was 0.3%, and an additional 135 patients (1.7%) were diagnosed with HCC over the next 27 months.
- We classified all subjects into four groups: a group without HCC, a group with HCC detected through the 1st surveillance USG test, a group with HCC detected within 12 months of the 1st surveillance USG test, and a group with HCC detected 12 months after the 1st surveillance USG test. The characteristics of the study groups are presented in Table 2. The subjects in the HCC detection group (those with HCC detected through the 1st surveillance USG test) were significantly older than those in the other groups (P<0.001). However, no significant difference in liver function or fibrosis burden was noted between the detection group and the later diagnosed groups (those with HCC detected within or after 12 months of the 1st surveillance USG test). Additionally, there was no significant difference between the detection and the later diagnosis groups in terms of examiner experience, USG duration, or USG machine manufacturer.
- 3. Characteristics of patients with HCC
- During the mean observation period of 27.2±9.7 months, 158 patients (2.0%) developed HCC. The characteristics of the groups with versus without HCC are compared in Table 3. The proportion of males in the HCC group (65.8%) was higher than that in the non-HCC group (56.5%; P=0.024), and the subjects in the HCC group were significantly older (mean, 61.4 years) than those in the non-HCC group (mean, 53.4 years; P<0.001). Additionally, a higher proportion of patients was taking antiviral drugs in the HCC versus nonHCC group. However, there was no significant difference in the incidence of HCC according to etiology (P=0.083).
- The fibrosis burden was higher in the HCC versus non-HCC group when evaluated using FIB-4 score, APRI score, or transient elastography. In addition, subjects in the HCC group were significantly more likely to have liver cirrhosis (74.7%) than those in the non-HCC group (27.9%; P<0.001). Meanwhile, a significantly lower proportion of subjects had fatty liver disease in the HCC (6.3%) versus non-HCC group (29.7%; P<0.001). In contrast to patientrelated factors, there were no differences in physician- or machine-related factors between the HCC and non-HCC groups.
- 4. HCC characteristics by diagnostic timing
- Finally, HCC stage and characteristics were compared by HCC diagnostic timing (i.e., on the 1st surveillance USG test, within 12 months after the 1st surveillance USG test, and 12 months after the 1st surveillance USG test) (Table 4). Regarding tumor location, the right lobe was most commonly affected, accounting for an average of 62%, than the left lobe; this trend did not differ significantly among groups. The average HCC size was 2.4±2.0 cm, and no intergroup difference was noted. Most HCC cases were hypoechoic on USG, with the nodular type being the most prevalent. The most common HCC stage was Barcelona Clinic Liver Cancer (BCLC) stage A (87%), followed by BCLC stage B (7%). An additional stratified analysis was performed according to liver disease etiology (viral vs. non-viral), but no difference was found (data not shown). In summary, no significant differences in HCC stage or characteristics were noted at the time of diagnosis.
RESULTS
- This study provides insight into the current status of screening tests for high-risk HCC groups in South Korean tertiary hospitals. The most important factors in detecting HCC were patient-related, whereas the effects of physician-or machine-related factors were not significant. Additionally, case classification based on the time from the 1st screening USG to HCC diagnosis suggested a low likelihood of missing HCC on USG and satisfactory performance of South Korean surveillance testing.
- The importance of USG in liver cancer screening cannot be overemphasized. Abdominal USG is strongly recommended as a surveillance test for HCC regardless of the national guidelines.8-10 In fact, it is widely recognized that regular screening using USG can increase survival rates and reduced medical expenses for patients at a high risk of HCC.11 In South Korea, an early cancer screening program was implemented for high-risk groups for HCC in 2003; however, few studies have focused on the specific procedures of USG examinations in clinical practice.12
- The primary significance of this study is that it sheds light on the current status of HCC surveillance in South Korea. While national annual surveillance data were published in 2016,11 this is the first study to examine how HCC screening is conducted at tertiary hospitals in the South Korean metropolitan area. In South Korean tertiary hospitals, gastroenterologists actively participate in HCC screening, whereas globally, surveillance USG is primarily performed by radiology departments.13 In the long term, it will be necessary to pay much attention to USG education in gastroenterology. Regular visits with specialists, such as hepatologists, can effectively increase patient adherence. In the United States and Europe, <30% of patients with liver cirrhosis undergo regular USG examinations, but the screening rate significantly increased when a specialist was consulted.14,15
- The second important finding of our study was that the likelihood of HCC being missed by USG was low. We classified HCC cases based on the timing of diagnosis since the 1st surveillance USG and found no significant intergroup differences. It is generally understood that the doubling time for HCC is 6–12 months given that we defined the cases of an additional HCC discovered within 12 months since the 1st surveillance USG as possible instances of missed HCC cases.16 If this group truly represented missed HCC cases, we would expect to observe a higher HCC stage compared to the group in which HCC was diagnosed at the 1st surveillance USG. However, our results did not support this hypothesis. That is, the group of subjects who had HCC detected within 12 months of the 1st surveillance USG did not have HCC at the time of the 1st surveillance, while it is highly likely that new HCC developed later. In summary, our findings suggest that current surveillance protocols for HCC in South Korean tertiary hospitals are unlikely to miss cases of HCC, and USG screening appears effective.
- The third important finding of our study was that both physician- and machine-related factors had a minimal effect on HCC detection. We examined these factors since they are modifiable, unlike patient-related factors. For example, if USG scans were required to last for a certain duration akin to colonoscopy withdrawal time, this could be clinically implemented. However, we did not identify any readily modifiable physician- or machine-related factors in our study. To increase inspection accuracy, the development and control of quality indicators are important. Except for the quality indicators for the machine itself, there has been little research on indicators for performers.17 In 2021, a study evaluated performance evaluation criteria for abdominal USG in China;18 at that time, the items were doctor-to-USG equipment ratio, doctor-to-patient ratio, examination room-to-examination ratio, doctor-to-USG equipment ratio, and positive USG examination rate. Since the creation of these evaluation criteria, the diagnostic accuracy of abdominal USG has increased. However, there has been no research on the quality indicators of abdominal USG, particularly in Korea.
- Regarding the time taken for USG examination, a current duration of 12 minutes is appropriate for HCC detection. Notably, our results showed that experts with more than 15 years of experience achieved similar clinical results despite spending relatively less time on the examination than those with less than 15 years of experience. Regarding tumor factors, there were relatively few cases of fatty liver disease in patients with HCC. It is possible that the presence or absence of fatty liver influenced HCC detection; however, this hypothesis requires further validation. Likewise, although not statistically significant, the proportion of the infiltrative type in the missing HCC group was higher than that in the other HCC groups. As the infiltrative tumor type corresponds to a difficult form of HCC detection through USG, clinical attention is required.
- One of our study’s objectives was to develop a quality indicator for USG screening, but we were unable to identify any criteria for physician- or machine-related factors. In conclusion, patient-related factors remained the most important contributors to HCC development and detection. Among patient-related factors, advanced fibrosis burden, male sex, and older age were key risk factors, consistent with previous studies.19,20
- Although not identified in our study, the development of quality indicators for USG screening remains an important issue in the long term.21,22 This is because USG is currently the most widely used tool for HCC surveillance.23 However, USG has inherent limitations in terms of sensitivity, with a rate of 37%.5 Moreover, USG sensitivity tends to decline even further in patients with fatty liver or liver cirrhosis.24-26 Considering the increasing incidence of obesity and fatty liver, it is important to consider whether additional tests such as liquid biopsy should be performed in these patients in addition to surveillance USG.27-29
- Our study had several limitations. First, the definition of a group with the possibility of missed HCC lacks clear empirical evidence. In previous studies, surveillance failure was often defined by tumor stage, such as beyond the Milan criteria or at BCLC stage B or C.30 However, since the above definition may classify fast-growing HCC as surveillance failure, in our study, missing HCC was defined based on a 12-month interval. The doubling time for liver cancer varies greatly among individuals, so there may be objections to the criterion of <12 months used in our study.31 However, although not presented in this study, we performed a sensitivity analysis by changing the criteria in cases in which additional HCC was found within 6 months after the 1st surveillance USG; however, no significant difference was found across groups. Second, since our study mainly investigated USG practices at tertiary hospitals, there is a possibility of selection bias, and it may not represent surveillance practices in South Korea as a whole. In other words, to identify a comprehensive range of quality indicators of USG surveillance, various healthcare settings, including primary medical institutions and university hospitals, should have been included. Moreover, it is possible that the effects of physician- and machine-related factors were underestimated because the data were collected from university hospitals with relatively experienced physicians. It is probable that the proficiency of physicians or machines in primary medical institutions is lower than that in university hospitals, and future research targeting a broader range of hospitals is required.
- In conclusion, the present study conducted at South Korean tertiary hospitals in the metropolitan area showed that the current HCC detection rate of screening was 0.3%. In HCC detection, the influence of physician- or machine-related factors was minimal, while patient-related factors were more important. The possibility of missed HCC on USG was low, and HCC screening USG appeared to be performed in a clinically appropriate manner. Follow-up studies targeting a broader range of hospitals are needed to develop USG quality indicators in the future.
DISCUSSION
-
Conflict of Interest
Do Seon Song, Young Chang and Jeong-Ju Yoo currently serve as editorial board members for J Liver Cancer, and they were not involved in the review process of this article. Otherwise, the authors have no conflicts of interest to disclose.
-
Ethics Statement
The study protocol was approved by the institutional review board (IRB) of each hospital (SCHBC 2019-09-009-001; date of registration: December 26, 2019) and conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki. Each IRB waived the requirement for informed consent owing to the retrospective nature of the study.
-
Funding Statement
This study was supported by the Soonchunhyang University research fund.
-
Data Availability
The data presented in this study are available upon request from the corresponding authors.
-
Author Contribution
Conceptualization: SHY, SSK, JJY
Formal analysis: JJY
Investigation: SGK, JHK, HAL, YSS, YKJ, HJY, DSS, SHK, MYK, YHA, JH, YSK, YC, SWJ, JYJ
Writing–original draft: SHY, SSK, JJY
Writing–review & editing: SHY, JJY
Approval of final manuscript: all authors
Article information
Supplementary Material

- 1. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182−236.ArticlePubMed
- 2. Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol 2020;72:250−261.PubMedPMC
- 3. Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a metaanalysis. Gastroenterology 2018;154:1706−1718.e1.ArticlePubMedPMC
- 4. Yoon JS, Lee HA, Kim HY, Sinn DH, Lee DH, Hong SK, et al. Hepatocellular carcinoma in Korea: an analysis of the 2015 Korean nationwide cancer registry. J Liver Cancer 2021;21:58−68.ArticlePubMedPMCPDF
- 5. Esfeh JM, Hajifathalian K, Ansari-Gilani K. Sensitivity of ultrasound in detecting hepatocellular carcinoma in obese patients compared to explant pathology as the gold standard. Clin Mol Hepatol 2020;26:54−59.ArticlePubMedPMCPDF
- 6. Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006;355:2533−2541.ArticlePubMed
- 7. Korean Liver Cancer Association (KLCA), National Cancer Center (NCC). 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. J Liver Cancer 2022 Dec 9. doi: 10.17998/jlc.2022.11.07. [Epub ahead of print].ArticlePDF
- 8. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723−750.PubMed
- 9. Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, et al. Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res 2019;49:1109−1113.PubMed
- 10. Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. AsiaPacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317−370.ArticlePubMedPDF
- 11. Kwon JW, Tchoe HJ, Lee J, Suh JK, Lee JH, Shin S. The impact of national surveillance for liver cancer: results from real-world setting in Korea. Gut Liver 2020;14:108−116.PubMed
- 12. Sohn W, Lee YS, Lee JG, An J, Jang ES, Lee DH, et al. A survey of liver cancer specialists’ views on the National Liver Cancer screening program in Korea. J Liver Cancer 2020;20:53−59.ArticlePubMedPMCPDF
- 13. da Silva PH, Gomes MM, de Matos CAL, de Souza E Silva IS, Gonzalez AM, Torres US, et al. HCC detection on surveillance US: comparing focused liver protocol using US LI-RADS technical guidelines to a general complete abdominal US protocol. J Ultrasound Med 2021;40:2487−2495.ArticlePubMedPDF
- 14. Singal AG, Li X, Tiro J, Kandunoori P, Adams-Huet B, Nehra MS, et al. Racial, social, and clinical determinants of hepatocellular carcinoma surveillance. Am J Med 2015;128:90.e1−e7.PubMed
- 15. Mehta NJ, Celik AD, Peters MG. Screening for hepatocellular carcinoma: what is missing? Hepatol Commun 2016;1:18−22.ArticlePubMedPMCPDF
- 16. Nathani P, Gopal P, Rich N, Yopp A, Yokoo T, John B, et al. Hepatocellular carcinoma tumour volume doubling time: a systematic review and meta-analysis. Gut 2021;70:401−407.ArticlePubMed
- 17. Kim DM, Park SK, Park SG. A study on the performance evaluation criteria and methods of abdominal ultrasound devices based on international standards. Safety 2021;7:31. Article
- 18. Tao X, Li J, Gu Y, Ma L, Xu W, Wang R, et al. A national quality improvement program on ultrasound department in China: a controlled cohort study of 1297 public hospitals. Int J Environ Res Public Health 2022;20:397. PubMedPMC
- 19. Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol 2008;14:4300−4308.PubMedPMC
- 20. Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol 2018;68:526−549.PubMed
- 21. Tanaka H. Current role of ultrasound in the diagnosis of hepatocellular carcinoma. J Med Ultrason (2001) 2020;47:239−255.ArticlePubMedPMCPDF
- 22. Simmons O, Fetzer DT, Yokoo T, Marrero JA, Yopp A, Kono Y, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther 2017;45:169−177.ArticlePubMedPDF
- 23. Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 2009;30:37−47.ArticlePubMedPMC
- 24. Lupsor-Platon M, Serban T, Silion AI, Tirpe GR, Tirpe A, Florea M. Performance of ultrasound techniques and the potential of artificial intelligence in the evaluation of hepatocellular carcinoma and non-alcoholic fatty liver disease. Cancers (Basel) 2021;13:790. PubMedPMC
- 25. Kim DH, Choi JI. Current status of image-based surveillance in hepatocellular carcinoma. Ultrasonography 2021;40:45−56.PubMed
- 26. Del Poggio P, Olmi S, Ciccarese F, Di Marco M, Rapaccini GL, Benvegnù L, et al. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:1927−1933.e2.ArticlePubMed
- 27. Sumida Y, Yoneda M, Seko Y, Ishiba H, Hara T, Toyoda H, et al. Surveillance of hepatocellular carcinoma in nonalcoholic fatty liver disease. Diagnostics (Basel) 2020;10:579. ArticlePubMedPMC
- 28. Plaz Torres MC, Bodini G, Furnari M, Marabotto E, Zentilin P, Strazzabosco M, et al. Surveillance for hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: universal or selective? Cancers (Basel) 2020;12:1422. ArticlePubMedPMC
- 29. Choi EJ, Kim YJ. Liquid biopsy for early detection and therapeutic monitoring of hepatocellular carcinoma. J Liver Cancer 2022;22:103−114.ArticlePubMedPMCPDF
- 30. Kim YY, An C, Kim DY, Aljoqiman KS, Choi JY, Kim MJ. Failure of hepatocellular carcinoma surveillance: inadequate echogenic window and macronodular parenchyma as potential culprits. Ultrasonography 2019;38:311−320.ArticlePubMedPMCPDF
- 31. An C, Choi YA, Choi D, Paik YH, Ahn SH, Kim MJ, et al. Growth rate of early-stage hepatocellular carcinoma in patients with chronic liver disease. Clin Mol Hepatol 2015;21:279−286.ArticlePubMedPMCPDF
References
Figure & Data
References
Citations

- The Epidemiology of Hepatitis B Virus Infection in Korea: 15-Year Analysis
Log Young Kim, Jeong-Ju Yoo, Young Chang, Hoongil Jo, Young Youn Cho, Sangheun Lee, Dong Hyeon Lee, Jae Young Jang
Journal of Korean Medical Science.2024;[Epub] CrossRef - Long-Term HBsAg Titer Kinetics with Entecavir/Tenofovir: Implications for Predicting Functional Cure and Low Levels
Soon Kyu Lee, Soon Woo Nam, Jeong Won Jang, Jung Hyun Kwon
Diagnostics.2024; 14(5): 495. CrossRef

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION
 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation

 Follow JLC on Twitter
Follow JLC on Twitter