Articles
- Page Path
- HOME > J Liver Cancer > Volume 23(1); 2023 > Article
-
Original Article
Subclassification of advanced-stage hepatocellular carcinoma with macrovascular invasion: combined transarterial chemoembolization and radiotherapy as an alternative first-line treatment -
Sujin Jin1
 , Won-Mook Choi1
, Won-Mook Choi1 , Ju Hyun Shim1
, Ju Hyun Shim1 , Danbi Lee1
, Danbi Lee1 , Kang Mo Kim1
, Kang Mo Kim1 , Young-Suk Lim1
, Young-Suk Lim1 , Han Chu Lee1
, Han Chu Lee1 , Jinhong Jung2
, Jinhong Jung2 , Sang Min Yoon2
, Sang Min Yoon2 , Jonggi Choi1
, Jonggi Choi1
-
Journal of Liver Cancer 2023;23(1):177-188.
DOI: https://doi.org/10.17998/jlc.2023.03.04
Published online: March 23, 2023
1Department of Gastroenterology, Liver Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
2Department of Radiation Oncology, Liver Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
-
Corresponding author: Jonggi Choi, Department of Gastroenterology, Liver Center, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea
Tel. +82-2-3010-1328, Fax. +82-2-485-5782 E-mail: j.choi@amc.seoul.kr
© 2023 The Korean Liver Cancer Association.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,920 Views
- 96 Downloads
- 3 Citations
Abstract
-
Background/Aim
- The Barcelona Clinic Liver Cancer (BCLC) guidelines recommend systemic therapy as the only first-line treatment for patients with BCLC stage C hepatocellular carcinoma (HCC) despite its heterogeneity of disease extent. We aimed to identify patients who might benefit from combined transarterial chemoembolization (TACE) and radiation therapy (RT) by subclassifying BCLC stage C.
-
Methods
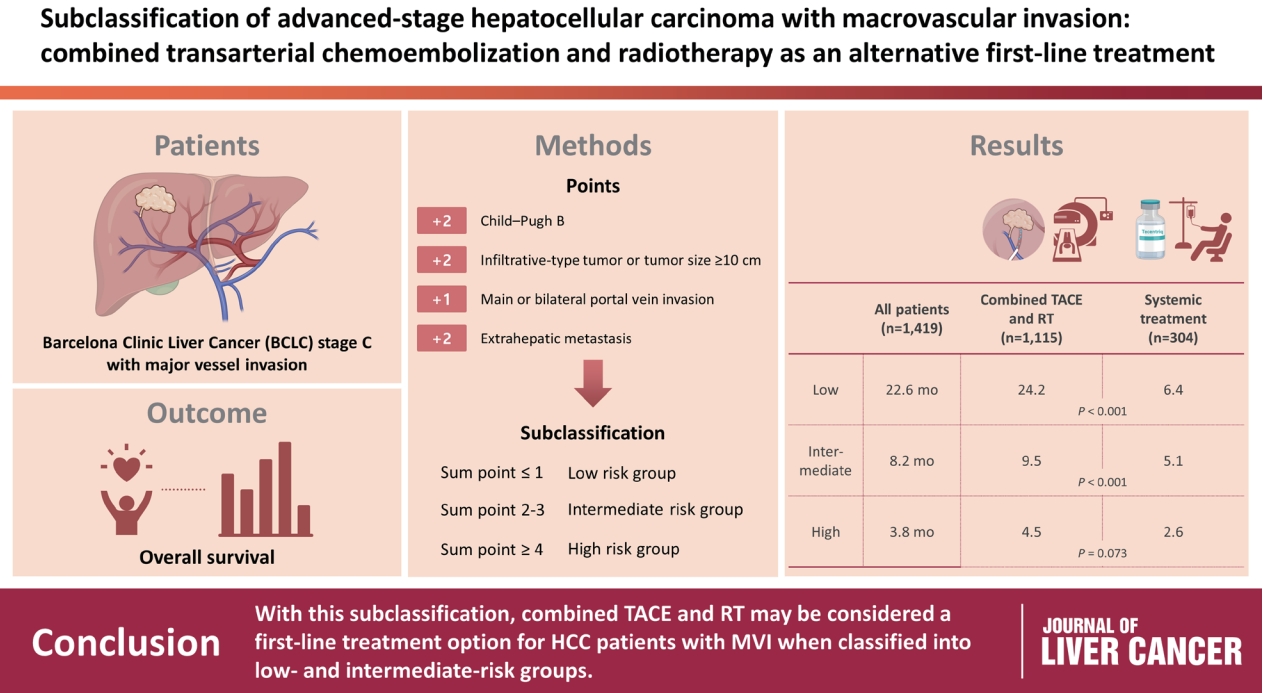
- A total of 1,419 treatment-naïve BCLC stage C patients with macrovascular invasion (MVI) who were treated with combined TACE and RT (n=1,115) or systemic treatment (n=304) were analyzed. The primary outcome was overall survival (OS). Factors associated with OS were identified and assigned points by the Cox model. The patients were subclassified into three groups based on these points.
-
Results
- The mean age was 55.4 years, and 87.8% were male. The median OS was 8.3 months. Multivariate analysis revealed a significant association of Child-Pugh B, infiltrative-type tumor or tumor size ≥10 cm, main or bilateral portal vein invasion, and extrahepatic metastasis with poor OS. The sub-classification was categorized into low (point ≤1), intermediate (point=2), and high (point ≥3) risks based on the sum of points (range, 0–4). The OS in the low, intermediate, and high-risk groups was 22.6, 8.2, and 3.8 months, respectively. In the low and intermediate-risk groups, patients treated with combined TACE and RT exhibited significantly longer OS (24.2 and 9.5 months, respectively) than those who received systemic treatment (6.4 and 5.1 months, respectively; P<0.0001).
-
Conclusions
- Combined TACE and RT may be considered as a first-line treatment option for HCC patients with MVI when classified into low- and intermediate-risk groups.
- Liver cancer is the second leading cause of cancer-related mortality worldwide, with hepatocellular carcinoma (HCC) accounting for 90% of all liver cancer cases.1 The poor prognosis of HCC is mainly due to the fact that approximately half of HCC patients are diagnosed at an advanced stage.2 Thus, these patients have limited access to curative treatment.
- Owing to its extensive applicability and ability to predict prognosis, the Barcelona Clinic Liver Cancer (BCLC) stage has been widely used worldwide to achieve better prognostication and guide HCC treatment.3 Nevertheless, BCLC stage C comprises a variety of HCC disease extents, including HCC with macrovascular invasion (MVI), extrahepatic metastasis, and both MVI and metastasis. Although these may have distinct prognoses, the BCLC staging system only recommends systemic treatment for BCLC stage C HCC.3 In this regard, there have been concerns that BCLC stage C HCC may require a distinct approach and treatment strategy based on the disease extent thereof. For example, the current BCLC stage classifies the same stage in both HCC with branch portal vein invasion (PVI) and main PVI, despite these conditions having vastly different prognoses.
- Prior to the introduction of effective systemic treatment for HCC, BCLC stage C HCC was traditionally treated with various modalities in East Asia, including transarterial chemoembolization (TACE), surgical resection, and external beam radiation therapy (RT). As RT may decrease the extent of vascular invasion and result in the restoration of blood flow to the normal liver, combining RT with TACE may allow the preservation of liver function, and the addition of TACE sessions may assist in the management of primary intrahepatic HCC. Previous studies have demonstrated the efficacy and safety of combined TACE and RT in the treatment of HCC and MVI.4-10 Furthermore, a randomized controlled trial revealed that TACE with RT resulted in longer progression-free and overall survival (OS) than sorafenib.11 Recent phase 3 trials have shown that novel systemic treatments, such as atezolizumab with bevacizumab and tremelimumab with durvalumab, are more effective than sorafenib.12,13 In these trials, HCC with MVI had a poor outcome compared with HCC without MVI, even at the same stage, in other words, BCLC stage C. This suggests that there are still patients who would benefit more from combined TACE and RT than from systemic treatment, given the unsatisfactory efficacy of the latter.
- In this regard, we aimed to determine whether patients would benefit more from combined TACE and RT than from systemic treatment by subclassifying BCLC stage C HCC according to baseline patient characteristics, tumor characteristics, and liver functional reserve in a large-scale cohort of treatment-naïve patients with HCC.
INTRODUCTION
- 1. Study population
- We retrospectively analyzed 1,419 consecutive patients treated with either combined TACE and RT or systemic treatment as first-line therapy for HCC with MVI at the Asan Medical Center, Seoul, Republic of Korea, between 2009 and 2018. All de-identified data were obtained from the electronic medical records of Asan Medical Center. HCC diagnosis was made based on histology or typical imaging features, according to the international guidelines for HCC.14-16 The presence of MVI was assessed via four-phase dynamic computed tomography (CT) or magnetic resonance imaging (MRI) using the following criteria: an intraluminal filling defect close to the HCC in the portal and/or hepatic vein and/or inferior vena cava, and an arterial enhancement of this filling defect and a washout on the portal/delayed phase.
- The inclusion criteria for this study were as follows: 1) BCLC stage C, 2) presence of MVI on imaging modalities, 3) no history of previous treatments for HCC by any modality, 4) Child-Pugh (CP) classification A or B hepatic function, and 5) a European Cooperative Oncology Group performance status of 0–2. This study was approved by the Institutional Review Board of the Asan Medical Center (IRB No. 2019-0932), and the need for informed consent was waived due to the retrospective nature of the study. The strengthening the reporting of observational studies in epidemiology (STROBE) reporting guidelines were followed (Supplementary Table 1).
- 2. Treatment for the study population
- TACE was performed at our center, as previously described.17 Briefly, after selective catheterization of the feeding artery using a microcatheter, cisplatin or doxorubicin (50 mg) was infused into the feeding artery according to tumor location and volume for approximately 15 minutes. Embolization was performed using an emulsion of iodized oil (Lipiodol, Guerbet, Roissy, France) and gelfoam slurry (Upjohn, Kalamazoo, MI, USA) after infusion of chemotherapeutic agents. TACE without gelfoam was performed in a subset of patients according to the severity of portal vein blood flow impairment. TACE was mostly performed before RT within an interval of 4 weeks and was repeated on an on-demand basis every 6–10 weeks following the previous TACE if residual viable HCC was obvious on subsequent dynamic CT or MRI.
- The detailed RT method has been previously described.6,10,11 In brief, four-dimensional CT (GE LightSpeed RT 16; GE Healthcare, Waukesha, WI, USA) images were obtained during free breathing, and these images were synchronized with respiratory data, which were sorted into 10 CT series based on the respiratory phase (0–90%). The target volumes were delineated on end-exhale-phase CT images. Gross tumor volume (GTV) included vascular invasion and a 2 cm margin into the contiguous HCC in most patients with huge infiltrative HCC in the end-expiratory phase of CT imaging. The GTV included all HCC with MVI in patients with HCC. The internal target volume was defined as the sum of the individual GTV within the gated respiration phases. The planning target volume was expanded to include a 0.7 cm margin from the internal target volume. The planned total dose to the planning target volume was 45 Gy with a fraction size of 2.5–3.0 Gy using 6-, 10-, or 15-MV X-rays at five fractions per week with a linear accelerator (Varian Medical Systems, Palo Alto, CA, USA). A three-dimensional conformal RT technique was employed to determine radiation ports using a planning system (Eclipse ver. 10.0; Varian Medical System), and the actual beam delivery was performed using a respiratory-gating beam delivery technique.
- 3. Statistical analysis and method for subclassification
- Data are expressed as median with interquartile range (IQR) for continuous variables and numbers with percentages for categorical variables. T-test and chi-square or Fisher’s exact tests were used for continuous and categorical variables, respectively, as appropriate.
- The primary outcome of the present study was OS, defined as the time from the date of the first TACE to the date of death from any cause, or the last date of confirmed survival. Cumulative OS was computed using the Kaplan-Meier method. The log-rank test was used to compare the OS between patients who received combined TACE and RT and those who received systemic treatment. Univariate and multivariate Cox proportional hazards analyses were used to identify factors affecting OS.
- Factors associated with OS in multivariate analysis were used for patient subclassification. The nearest integer of the adjusted hazard ratio (AHR) from the multivariate analysis was used as the assigned score for the corresponding factor from 1 to 2. Subsequently, sub-classification into three groups (low, intermediate, and high) was performed using the sum of these scores. OS was compared between combined TACE and RT and systemic treatment in each group.
- For potential confounder minimization and fair comparison of prognosis between combined TACE and RT and systemic treatment, we also conducted propensity score (PS) matching analysis. PS was computed using 18 variables: sex, age, etiology of liver disease, platelet count, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, total bilirubin, albumin concentration, creatinine, prothrombin time, CP score, alpha-fetoprotein (AFP), tumor type (nodular or infiltrative), tumor number, maximum tumor size, presence of extrahepatic metastasis, and MVI location. A nearest-neighbor 1:1 or 1:2 matching scheme with a caliper size of 0.15 was used. All statistical analyses were conducted using R version 3.6.0, and a P<0.05 was considered statistically significant.
METHODS
- 1. Characteristics of the study population
- The characteristics of the study population are summarized in Table 1. Of the 1,419 patients, 1,241 (87.5%) were male, and their median age was 55.0 years. Hepatitis B infection (75.3%) was the most common cause of underlying liver disease. At baseline, 970 (68.4%) patients had CP class A, 979 (69.0%) had a single tumor, and 863 (60.8%) had a tumor size ≥10 cm or an infiltrative-type tumor. All patients included in this study had MVI, and 820 (57.8%) had unilateral branch MVI (either the portal or hepatic vein). Extrahepatic metastasis was observed in 218 patients (15.4 %). Combined TACE and RT was performed in 1,115 patients (78.6%), whereas 304 patients (21.4%) received systemic treatment, mostly sorafenib.
- 2. Overall survival and outcomes according to the first-line treatment
- During the median follow-up of 7.7 months (IQR, 3.6–19.6), 1,146 patients (80.8%) died, with a median OS of 8.3 months. The 1-, 2-, 3-, and 5-year survival rates in the entire study population were 40.8%, 24.4%, 14.8%, and 13.6%, respectively.
- The median OS of patients who received combined TACE and RT and systemic treatment was 10.7 and 3.9 months, respectively (P<0.001). The survival rates at 1, 2, 3, and 5 years in patients who received combined TACE and RT were 47.1%, 28.6%, 20.8%, and 16.2%, respectively. Patients who received systemic treatment had survival rates of 16.1%, 7.2%, 4.0%, and 2.7% at 1, 2, 3, and 5 years after treatment, respectively.
- 3. Factors associated with overall survival
- In the univariate analysis, CP class B (hazard ratio [HR], 2.10; 95% confidence interval [CI], 1.86–2.38), CP class A, larger tumor size (≥10 cm or infiltrative type; HR, 2.12; 95% CI, 1.87–2.39), main branch vascular invasion (HR, 1.49; 95% CI, 1.32–1.67), and extrahepatic metastasis (HR, 2.03; 95% CI, 1.74–2.38) were associated with poorer OS (Table 2).
- In the multivariate analysis, factors associated with OS were CP class B (AHR, 1.86; 95% CI, 1.64–2.10), tumor size ≥10 cm or infiltrative-type tumor (AHR, 1.86; 95% CI, 1.64–2.11), main or bilateral vascular invasion (AHR, 1.23; 95% CI, 1.09–1.39), and presence of extrahepatic metastasis (AHR, 1.89; 95% CI, 1.62–2.22) (Table 2).
- 4. Subclassification and overall survival
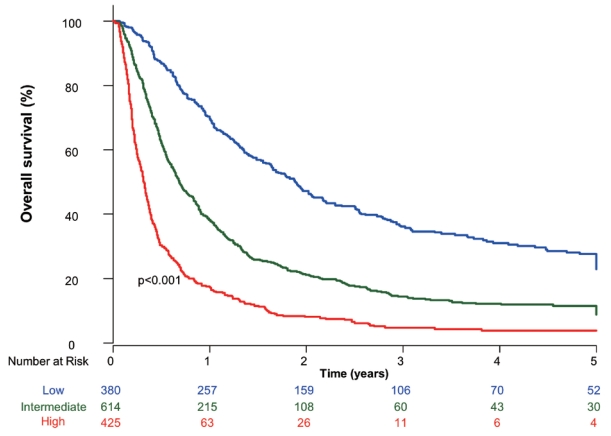
- Based on the AHR for each factor from the multivariate analysis, CP class B, tumor size ≥10 cm or infiltrative-type tumor, and presence of extrahepatic metastasis were each assigned a score of 2, whereas main or bilateral vascular invasion was assigned a score of 1. The sub-classification into three groups was based on the sum of these four scores, ranging from 0 to 7. Low (380 patients, 26.8%), intermediate (614 patients, 43.2%), and high (425 patients, 30.0%) risks were classified according to the ranges of 0–1, 2–3, and ≥4, respectively (Table 3). The median OS of the low, intermediate, and high-risk groups was 22.6, 8.2, and 3.8 months, respectively (Fig. 1).
- 5. Survival according to the first-line treatment based on the subclassification
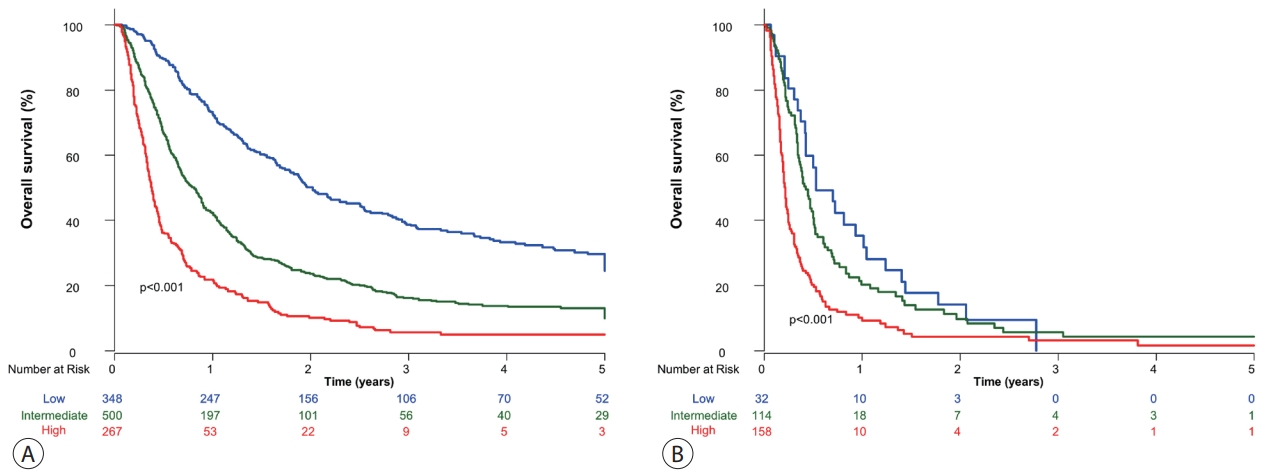
- Subclassification into low, intermediate, and high-risk groups showed statistically different OS in patients who received combined TACE and RT (Fig. 2A) and systemic treatment (Fig. 2B).
- Of the 380 patients in the low-risk group, 348 (91.6%) were treated with combined TACE and RT, whereas 32 (8.4%) received systemic treatment. The median OS of patients in the two groups differed significantly (combined TACE and RT, 24.2 months; systemic treatment, 6.4 months; P<0.001) (Fig. 3A). In the intermediate-risk group, the median OS was 9.5 months in patients who received combined TACE and RT (n=500), which was significantly longer than that in patients who received systemic treatment (n=114, 5.1 months, P<0.001) (Fig. 3B). Of the 425 patients in the high-risk group, those treated with combined TACE and RT had significantly longer OS than those who received systemic treatment (P<0.001) (Fig. 3C).
- 6. Propensity score-matching analysis
- Of the patients in the low-risk group, the median OS for patients who received combined TACE and RT and those who received systemic treatment was 19.9 and 8.4 months, respectively (Fig. 3D). Of the 107 PS-matched pairs in the intermediate-risk group, patients who received combined TACE and RT had a significantly longer median OS than those who received systemic treatment (8.2 vs. 5.4 months, P<0.001) (Fig. 3E). Among the high-risk group consisting of 70 PS-matched pairs, patients treated with combined TACE and RT, and those who received systemic treatment, had a median OS of 4.2 and 2.7 months, respectively (P=0.073) (Fig. 3F).
- 7. Subgroup analysis according to the presence of extrahepatic metastasis
- Of the 1,201 patients without extrahepatic metastasis, the median OS (11.5 months) in patients receiving TACE and RT was significantly longer than the median OS (4.2 months) in patients receiving systemic treatment (P<0.001) (Supplementary Fig. 1). Of the 218 patients with extrahepatic metastasis, the TACE and RT group showed a significantly longer OS than the systemic treatment group (4.6 vs 2.7 months, respectively) (Supplementary Fig. 2), despite the difference being less pronounced than that in patients without extrahepatic metastasis. In patients without extrahepatic metastasis, the median OS of the low, intermediate, and high-risk groups was 22.6, 8.3 and 3.9 months, respectively (Supplementary Fig. 3). Patients with extrahepatic metastasis were only included in the intermediate and high-risk groups, with a median OS of 6.2 and 3.7 months, respectively (Supplementary Fig. 4).
RESULTS
- In the present study of patients with HCC and MVI, those treated with combined TACE and RT had significantly longer OS than those who received systemic treatment. CP class B, main or bilateral vascular invasion, presence of extrahepatic metastasis, and tumor size ≥10 cm or infiltrative-type tumors were associated with a poor prognosis. Using these four factors, patients with HCC and MVI could be subclassified into three groups with distinct prognoses. Patients in the newly subclassified low-risk group who received combined TACE and RT had a median OS of 2 years, although they were still classified as BCLC stage C.
- A few studies have attempted to classify BCLC stage C HCC. A study involving 1,746 patients with BCLC stage C HCC, using registries from the Korean Liver Cancer Study Group, demonstrated that significant PVI, defined as PVI in the lobar, main, or bilateral branch, and extrahepatic metastasis, was associated with an increased risk of death.18 This finding is consistent with ours, resulting in the incorporation thereof in our subclassification system. However, unlike our study, that study did not provide detailed information on the treatment and prognosis of HCC. Another study analyzed 612 patients treated with sorafenib for BCLC stage C HCC.19 That study suggested a subclassification according to five prognostic factors: CP score, AFP, tumor type (nodular or infiltrative), extrahepatic metastasis, and PVI. Using these variables, the authors classified patients with BCLC stage C HCC into three groups, which clearly showed different OS. Notably, this study assigned greater points to CP class B (7) and AFP levels (3–10) rather than PVI (1) and extrahepatic metastasis (1) to give a maximum total of 24 points. Although all factors affecting poorer prognosis, except AFP levels, were consistent with those in our study, the magnitudes of each factor were significantly different from those in our study. Our study showed a similar impact of CP class and extrahepatic metastasis on prognosis compared to the previous study. This discrepancy may have been due to the differences in patient characteristics. The aforementioned study included most treatment-experienced patients who had received systemic treatment. However, our study only included treatment-naïve patients to demonstrate unbiased treatment effects of either combined TACE and RT or systemic treatment for BCLC stage C HCC.
- We previously suggested the use of a sub-classification model in patients who received combined TACE and RT for HCC with MVI.10 Of the 639 included patients, CP class B, extrahepatic metastasis, main or bilateral vascular invasion, and tumor size ≥10 cm or infiltrative-type tumors were associated with poor OS.10 The patients were then categorized into four groups (very low, low, intermediate, and high risk) based on these four factors. In the very low-risk group, without all four factors, patients who received combined TACE and RT had a median OS of 84 months, indicating a significantly better prognosis, given that the median survival of patients with HCC and MVI is considered to be approximately 9 to 12 months. However, in our previous study, we were unable to validate whether this subclassification is still applicable to the prediction of prognosis in patients who received systemic treatment. Furthermore, we did not compare OS between patients who received combined TACE and RT and those who received systemic treatment according to this subclassification. Thus, the present study overcomes these drawbacks and further modifies the sub-classification. In the present study, patients in the low-risk group who received combined TACE and RT as first-line treatment had a median OS of 24 months. However, those who received systemic treatment had a median OS of 6.4 months although they were also classified into the low-risk group. This suggests that a subset of HCC patients with MVI, despite having BCLC stage C, can benefit more from combined TACE and RT than from systemic treatment if they are classified into a low-risk group based on our subclassification.
- Many previous studies have demonstrated that the combination of RT and TACE is effective and prolongs the OS of patients with MVI.6,8,10,11 MVI, especially PVI, may impede portal flow into the liver, resulting in decreased liver function, thus, further treatment for HCC may not be allowed because of poor liver reserve, despite the existence of effective treatment. However, RT can reduce the restoration of portal flow by decreasing tumor thrombus in the portal vein, and TACE can control the intrahepatic tumor burden. In this regard, previous cohort studies, including randomized trials, have shown a more significant survival benefit from combined TACE and RT than from systemic treatment.10
- Almost all patients who received systemic treatment in this study were treated with sorafenib, as it was the only approved first-line treatment available during the study period. However, atezolizumab and bevacizumab treatment recently showed significantly better OS than sorafenib in the IMbrave150 trial.12 This implies that patients who will receive atezolizumab and bevacizumab treatments can anticipate much better OS in each risk group than the actual OS in the present study. Indeed, an updated analysis of the IMbrave150 trial revealed that patients with MVI had an OS of 14.2 months compared with those treated with sorafenib in the SHARP trial (OS of 8.1 months).20,21 Therefore, future studies should compare the efficacy between combined TACE and RT and newly approved systemic treatments, including atezolizumab and bevacizumab, and validate whether our subclassification is still applicable to patients who will receive new systemic treatments to overcome the limitation of our study.
- Additionally, patients with MVI have been shown to benefit from transarterial radioembolization (TARE) recently. Therefore, the performance of our proposed sub-classification in predicting the prognosis of TARE-treated patients should be further evaluated.
- Our study has several limitations. Considering its retrospective nature, it was inevitably subject to selection bias. Nevertheless, we included consecutive treatment-naïve patients to minimize bias. Furthermore, our study was conducted in a single center where combined TACE and RT was widely used as an effective treatment modality for patients with BCLC stage C HCC. However, other treatment modalities such as hepatic arterial infusion chemotherapy and concurrent chemoradiation therapy have also been used in other centers. Therefore, our subclassification may not be generalizable to patients who receive these treatments.
- In conclusion, combined TACE and RT had significantly better OS than systemic treatment in patients with HCC and MVI. Although all patients had BCLC stage C HCC, subclassification using four factors, namely, CP class B, extrahepatic metastasis, tumor size ≥10 cm or infiltrative-type tumor, and main or bilateral vascular invasion, resulted in a distinctive prognosis. A subset of patients with BCLC stage C HCC, particularly those in the low-risk group in our subclassification, can expect better survival if treated with combined TACE and RT.
DISCUSSION
Acknowledgments
-
Conflict of Interest
There are no other disclosures to declare. J Choi has served as a speaker and an advisory committee member for Gilead Sciences.
-
Ethics Statement
This study was approved by the Institutional Review Board of the Asan Medical Center (IRB No. 2019-0932), and the need for informed consent was waived due to the retrospective nature of the study.
-
Funding Statement
This study was supported by the Korean Liver Cancer Association Research Award (2021), and the National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science and ICT) (No. 2021R1G1A1009506).
-
Data Availability
The data presented in this study are available from the corresponding author upon reasonable request.
-
Author Contribution
Conceptualization: SJ, WK, JHS, DL, KMK, YL, HCL, JJ, SMY, JC
Data curation: SJ, WK, JC
Formal analysis: SJ, JC
Funding acquisition: JC
Investigation: JC
Methodology: SJ, JC
Resources: JHS, JC
Supervision: WK, JHS, DL, YL, HCL, JJ, SMY, JC
Validation: SJ, JC
Visualization: JC
Writing–original draft: SJ, JC
Writing–review & editing: SJ, WK, JHS, DL, KMK, YL, HCL, JJ, SMY, JC
Approval of the final version of the manuscript: all authors
Article information
Supplementary Material

- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394−424.ArticlePubMedPDF
- 2. Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 1999;29:62−67.ArticlePubMed
- 3. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, GarciaCriado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 2022;76:681−693.ArticlePubMed
- 4. Tazawa J, Maeda M, Sakai Y, Yamane M, Ohbayashi H, Kakinuma S, et al. Radiation therapy in combination with transcatheter arterial chemoembolization for hepatocellular carcinoma with extensive portal vein involvement. J Gastroenterol Hepatol 2001;16:660−665.PubMed
- 5. Liu PH, Lee YH, Hsia CY, Hsu CY, Huang YH, Chiou YY, et al. Surgical resection versus transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. Ann Surg Oncol 2014;21:1825−1833.ArticlePubMedPDF
- 6. Kim GA, Shim JH, Yoon SM, Jung J, Kim JH, Ryu MH, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol 2015;26:320−329.e6.ArticlePubMed
- 7. Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol 2016;65:938−943.ArticlePubMed
- 8. Yu JI, Park JW, Park HC, Yoon SM, Lim DH, Lee JH, et al. Clinical impact of combined transarterial chemoembolization and radiotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: an external validation study. Radiother Oncol 2016;118:408−415.ArticlePubMed
- 9. Lee D, Lee HC, An J, Shim JH, Kim KM, Lim YS, et al. Comparison of surgical resection versus transarterial chemoembolization with additional radiation therapy in patients with hepatocellular carcinoma with portal vein invasion. Clin Mol Hepatol 2018;24:144−150.ArticlePubMedPMCPDF
- 10. Kim YJ, Jung J, Joo JH, Kim SY, Kim JH, Lim YS, et al. Combined transarterial chemoembolization and radiotherapy as a first-line treatment for hepatocellular carcinoma with macroscopic vascular invasion: necessity to subclassify Barcelona Clinic Liver Cancer stage C. Radiother Oncol 2019;141:95−100.ArticlePubMed
- 11. Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol 2018;4:661−669.ArticlePubMedPMC
- 12. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894−1905.ArticlePubMed
- 13. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid 2022;1:1−12.Article
- 14. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182−236.ArticlePubMed
- 15. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358−380.ArticlePubMedPDF
- 16. Korean Liver Cancer Association, National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver 2019;13:227−299.ArticlePubMedPMC
- 17. Choi J, Lee D, Shim JH, Kim KM, Lim YS, Lee YS, et al. Evaluation of transarterial chemoembolization refractoriness in patients with hepatocellular carcinoma. PLoS One 2020;15:e0229696.PubMedPMC
- 18. Lee S, Kim BK, Song K, Park JY, Ahn SH, Kim SU, et al. Subclassification of Barcelona Clinic Liver Cancer B and C hepatocellular carcinoma: a cohort study of the multicenter registry database. J Gastroenterol Hepatol 2016;31:842−847.ArticlePubMedPDF
- 19. Yoo JJ, Chung GE, Lee JH, Nam JY, Chang Y, Lee JM, et al. Sub-classification of advanced-stage hepatocellular carcinoma: a cohort study including 612 patients treated with sorafenib. Cancer Res Treat 2018;50:366−373.ArticlePubMedPDF
- 20. Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821−829.ArticlePubMed
- 21. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 2022;76:862−873.ArticlePubMed
References
Figure & Data
References
Citations

- Liver resection in selective hepatocellular carcinoma with Vp3 or Vp4 portal vein tumor thrombosis improves prognosis
Manuel Lim, Jongman Kim, Jinsoo Rhu, Gyu-Seong Choi, Jae-Won Joh
Journal of Liver Cancer.2024; 24(1): 102. CrossRef - Comparison of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma with portal vein tumor thrombosis
Jeayeon Park, Yun Bin Lee, Yunmi Ko, Youngsu Park, Hyunjae Shin, Moon Haeng Hur, Min Kyung Park, Dae-Won Lee, Eun Ju Cho, Kyung-Hun Lee, Jeong-Hoon Lee, Su Jong Yu, Tae-Yong Kim, Yoon Jun Kim, Tae-You Kim, Jung-Hwan Yoon
Journal of Liver Cancer.2024; 24(1): 81. CrossRef - How to optimize the treatment strategy for advanced-stage hepatocellular carcinoma with macrovascular invasion
Beom Kyung Kim
Journal of Liver Cancer.2023; 23(1): 121. CrossRef

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION
 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation

 Follow JLC on Twitter
Follow JLC on Twitter