Search
- Page Path
- HOME > Search
Case Reports
- A case of nearly complete response in hepatocellular carcinoma with disseminated lung metastasis by combination therapy of nivolumab and ipilimumab after treatment failure of atezolizumab plus bevacizumab
- Hyung Jun Kim, Sang Youn Hwang, Jung Woo Im, Ki Jeong Jeon, Wan Jeon
- J Liver Cancer. 2023;23(1):213-218. Published online March 9, 2023
- DOI: https://doi.org/10.17998/jlc.2023.02.23

- 1,266 Views
- 74 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
PDF - Recently, the efficacy of immuno-oncologic agents for advanced hepatocellular carcinoma (HCC) has been proven in several trials. In particular, atezolizumab with bevacizumab (AteBeva), as a first-line therapy for advanced HCC, has shown tremendous advances in the IMBrave150 study. However, second or third-line therapy after treatment failure with AteBeva has not been firmly established. Moreover, clinicians have continued their attempts at multidisciplinary treatment that includes other systemic therapy and radiotherapy (RT). Here, we report a case that showed a near complete response (CR) of lung metastasis to nivolumab with ipilimumab therapy after achieving a near CR of intrahepatic tumor using sorafenib and RT in a patient with advanced HCC who had experienced treatment failure of AteBeva.
-
Citations
Citations to this article as recorded by- Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline Update
John D. Gordan, Erin B. Kennedy, Ghassan K. Abou-Alfa, Eliza Beal, Richard S. Finn, Terence P. Gade, Laura Goff, Shilpi Gupta, Jennifer Guy, Hang T. Hoang, Renuka Iyer, Ishmael Jaiyesimi, Minaxi Jhawer, Asha Karippot, Ahmed O. Kaseb, R. Kate Kelley, Jerem
Journal of Clinical Oncology.2024; 42(15): 1830. CrossRef
- Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline Update

- Infiltrative hepatocellular carcinoma with multiple lung metastasis completely cured using nivolumab: a case report
- Ji Eun Han, Hyo Jung Cho, Soon Sun Kim, Jae Youn Cheong
- J Liver Cancer. 2021;21(2):169-176. Published online September 30, 2021
- DOI: https://doi.org/10.17998/jlc.2021.08.26

- 3,714 Views
- 83 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
PDF - The current Food and Drug Administration-approved systemic treatments for advanced hepatocellular carcinoma (HCC) include multikinase inhibitors (tyrosine kinase inhibitor [TKI]) and immune checkpoint inhibitors (ICIs). Among ICIs, nivolumab is used as secondline therapy for advanced HCC after sorafenib failure or patient intolerance. In this case, a patient with infiltrative HCC and portal vein tumor thrombosis was treated with hepatic arterial infusion chemotherapy (HAIC) and radiation therapy. New lung metastasis developed after HAICs; thus, lenvatinib treatment was initiated. However, the disease progressed. Thereafter, sorafenib treatment was initiated but he developed intolerance, with grade 3 sorafenib-related diarrhea. Subsequently, nivolumab was administered as rescue therapy. He demonstrated a partial response to nivolumab after the third treatment and viable HCCs in the lungs and liver completely disappeared after the 24th treatment. These findings suggest that nivolumab could be used as an effective rescue therapy for advanced HCC progression after TKI treatment.
-
Citations
Citations to this article as recorded by- Intermediate-stage (BCLC stage B) infiltrative hepatocellular carcinoma: safety and efficacy of chemoembolization
Seong Ho Kim, Jin Hyoung Kim, Gun Ha Kim, Ji Hoon Kim, Heung-Kyu Ko, Hee Ho Chu, Ji Hoon Shin, Dong Il Gwon, Gi-Young Ko, Hyun-Ki Yoon, Shakir Aljerdah, Nayoung Kim
European Radiology.2023; 33(12): 8736. CrossRef
- Intermediate-stage (BCLC stage B) infiltrative hepatocellular carcinoma: safety and efficacy of chemoembolization

- Nivolumab for Advanced Hepatocellular Carcinoma with Multiple Lung Metastases after Sorafenib Failure
- Jaewoong Kim, Jin Won Chang, Jun Yong Park
- J Liver Cancer. 2020;20(1):72-77. Published online March 31, 2020
- DOI: https://doi.org/10.17998/jlc.20.1.72
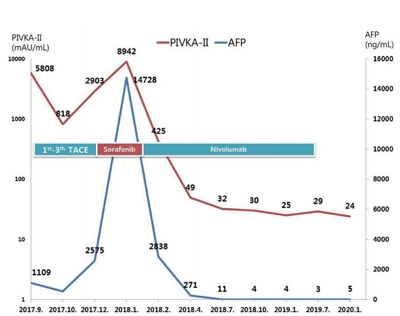
- 4,849 Views
- 143 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
PDF - Over the past decade, standard first-line systemic treatment of advanced hepatocellular carcinoma (HCC) has been based on sorafenib, a multi-kinase inhibitor. Regorafenib, another tyrosine kinase inhibitor, is the only second-line therapy that has been globally approved after progression under sorafenib treatment. Recently, immunotherapeutic agents have emerged as promising treatment options in many different malignancies, including advanced HCC. Nivolumab is the first immunotherapy approved by the Food and Drug Administration for use in HCC patients with advanced-stage second-line after sorafenib failure. In this report, a case of advanced HCC with multiple lung metastases in which a complete response and maintained progression-free status was achieved with nivolumab, following the failure of transarterial chemoembolization and sorafenib is presented. We hope this report may help expand the clinical application of second-line treatment.
-
Citations
Citations to this article as recorded by- Infiltrative hepatocellular carcinoma with multiple lung metastasis completely cured using nivolumab: a case report
Ji Eun Han, Hyo Jung Cho, Soon Sun Kim, Jae Youn Cheong
Journal of Liver Cancer.2021; 21(2): 169. CrossRef
- Infiltrative hepatocellular carcinoma with multiple lung metastasis completely cured using nivolumab: a case report

- A Case of Achieving Partial Remission with the Combination of Sorafenib and Nivolumab in a Patient with Hepatocellular Carcinoma Showing Disease Progression after Nivolumab Therapy
- Sang Youn Hwang, Seon-Mi Lee, Jung Woo Im, Ki Jeong Jeon, Cheol-Won Choi, Kyung-Su Kim, Wan Jeon
- J Liver Cancer. 2019;19(1):74-78. Published online March 31, 2019
- DOI: https://doi.org/10.17998/jlc.19.1.74
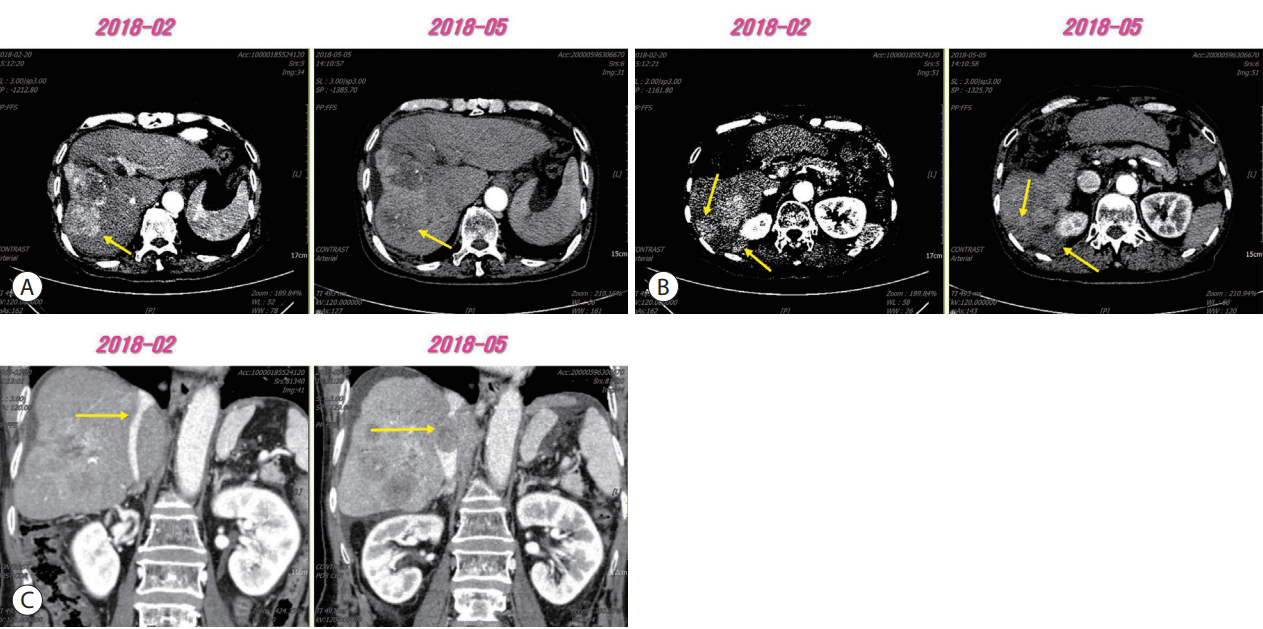
- 3,471 Views
- 51 Downloads
-
 Abstract
Abstract
 PDF
PDF - Sorafenib is a well-known approved systemic therapeutic agent used in patients with advanced hepatocellular carcinoma (HCC). Regorafenib and nivolumab are approved as second-line therapeutic drugs in patients showing disease progression after sorafenib therapy. However, there is no established third- or fourth-line therapy in patients with progression after regorafenib or nivolumab treatment. Recently, the combination of tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICPIs) has been attempted as a firstline treatment strategy in advanced HCC patients based on the hypothesis that combination therapy may overcome resistance in ICPI monotherapy. On the basis of this suggestion, we herein describe the case of an HCC patient demonstrating macrovascular invasion, whereby partial remission was achieved via the combination of sorafenib and nivolumab following disease progression after nivolumab therapy. Further studies on the combination of TKIs and ICPIs are necessary to determine ways to manage HCC patients showing disease progression after ICPI therapy.


 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION

 First
First Prev
Prev



 Follow JLC on Twitter
Follow JLC on Twitter