The diagnostic value of circulating tumor DNA in hepatitis B virus induced hepatocellular carcinoma: a systematic review and meta-analysis
Article information
Abstract
Background/Aim
New biomarkers are urgently needed to aid in the diagnosis of early stage hepatocellular carcinoma (HCC). We performed a meta-analysis on the diagnostic utility of circulating tumor DNA (ctDNA) levels in patients with hepatitis B virus-induced HCC.
Methods
We retrieved relevant articles from PubMed, Embase, and the Cochrane Library up to February 8, 2022. Two subgroups were defined; one subset of studies analyzed the ctDNA methylation status, and the other subset combined tumor markers and ctDNA assays. Pooled sensitivity (SEN), specificity (SPE), positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under the summary receiver operating characteristic curve (AUC) were analyzed.
Results
Nine articles including 2,161 participants were included. The overall SEN and SPE were 0.705 (95% confidence interval [CI], 0.629-0.771) and 0.833 (95% CI, 0.769-0.882), respectively. The DOR, PLR, and NLR were 11.759 (95% CI, 7.982-17.322), 4.285 (95% CI, 3.098-5.925), and 0.336 (0.301-0.366), respectively. The ctDNA assay subset exhibited an AUC of 0.835. The AUC of the combined tumor marker and ctDNA assay was 0.848, with an SEN of 0.761 (95% CI, 0.659-0.839) and an SPE of 0.828 (95% CI, 0.692-0.911).
Conclusions
Circulating tumor DNA has promising diagnostic potential for HCC. It can serve as an auxiliary tool for HCC screening and detection, especially when combined with tumor markers.
INTRODUCTION
In 2020, primary liver cancer was the sixth most common cancer and third leading cause of cancer-related deaths.1 Hepatocellular carcinoma (HCC) is the most common primary liver cancer (75-90% of all the cases).2 Of the several risk factors, chronic hepatitis B virus (HBV) infection is the most important, being associated with approximately 54% of HCC cases worldwide3 and 62.2% of HCC cases in Korea.4 Although early diagnosis is of utmost importance, HCC is often initially asymptomatic; most patients are diagnosed in the middle or late stages. Therefore, surveillance of patients with high-risk features on ultrasonography (US), with or without the measurement of α-fetoprotein (AFP) levels, is important.5,6 However, the recommended surveillance methods vary between studies. The sensitivity (SEN) of US-mediated early stage HCC detection in high-risk patients (with and without serum AFP measurement) was approximately 60%.7-9 New biomarkers that facilitate early detection are thus required. Circulating tumor DNA (ctDNA) fragment assays (also known as ‘liquid biopsies’) may aid HCC detection10,11 because ctDNA contains all of the genetic information of tumor cells and can be detected in the peripheral blood of early stage HCC patients.12,13 HCC development is accompanied by genetic and epigenetic mutations in the ctDNA. HCC-specific mutations in the plasma DNA have been reported in several studies.14-16 However, given the low amounts of ctDNA in patients with early stage cancers, it is difficult to distinguish true mutations from PCR and sequencing errors.17 A panel of common HCC-associated mutations has been used to diagnose HCC. Genomic profiling has identified key driver mutations in TP53, CTNNB1, PIK-3CA, PTEN, AXIN1, the promoter of TERT, and integrated HBV genomes.18-20 Both hyper and hypomethylated ctDNA regions are used for early tumor detection, and epigenetic changes play important roles in carcinogenesis.21,22 It is easier to detect, amplify, and quantify aberrant ctDNA hypermethylation than to document genomic mutations.23 In humans, many 5-hydroxymethylcytosine (5hmC) epigenetic markers are generated via oxidation of 5-methylcytosine by ten-eleven translocation enzymes.24 Such biomarkers aid in precision medicine because they reflect cancer gene regulation at the tissue level. Technical advances have made liquid biopsies convenient.25-28 However, although many studies have reported that HCC ctDNA assays are diagnostic, they vary extensively in terms of design and results. Therefore, there is an urgent need to assess the diagnostic utility of HCC ctDNA assays. Since the etiology of HCC could be one of the reasons for the heterogeneity among previous studies, we performed a systematic literature review and meta-analysis to assess the diagnostic utility of ctDNA assays in HBV-induced HCC.
METHODS
1. Search strategy and literature selection
The meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary Table 1).29 We systematically searched PubMed, Embase, and the Cochrane library. The language was limited to English and the search was limited to humans. The search terms were “circulating tumor DNA” OR “circulating DNA” OR “ctDNA” OR “plasma DNA” OR “serum DNA” OR “liquid biopsy” OR “cell free tumor DNA” OR “cell free nucleic acid” OR “circulating cell free nucleic acid” OR “circulating nucleic acid” OR “cell free DNA” AND “liver cancer” OR “hepatocellular carcinoma” OR “liver neoplasm” OR “hepatic carcinoma” OR “liver tumor” OR “liver cell carcinoma” OR “hepatocarcinoma” OR “hepatoma” OR “liver cell cancer” OR “hepatic cancer” OR “hepatic neoplasm” OR “hepatocellular cancer” OR “hepatocellular neoplasm” OR “hepatocellular tumor” OR “liver cell neoplasm” OR “HCC” AND “chronic hepatitis B” OR “chronic hepatitis B virus infection” OR “hepatitis B virus” OR “chronic HBV” OR “HBV infection” OR “hepatitis B infection.” We manually reviewed the reference lists and added the relevant articles.
2. Inclusion and exclusion criteria
The inclusion criteria were HBV-induced HCC, HCC ctDNA assay of serum or plasma, use of ctDNA data for initial HCC diagnosis, and availability of sensitivity (SEN) and specificity (SPE) data (either explicit or calculable). The exclusion criteria were reviews, cases, abstracts, letters, comments, meta-analyses, duplicate reports, predictions of HCC risk or prognosis, no SEN or SPE data, and/or analysis of ctDNAs that are not in the serum or plasma (rather in the liver or urine).
3. Data extraction and quality assessment
Two investigators independently selected relevant studies and summarized the results. We collected the following data: first author, publication year, references, study type, control modality, sample size, sampling time, sample source, detection methods, assay indicators, cutoff values, SEN, SPE, true positive (TP), true negative (TN), false positive (FP), and false negative (FN) values. Both investigators independently assessed the study quality using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 criteria30 and any disagreement was resolved via discussion.
4. Statistical analysis
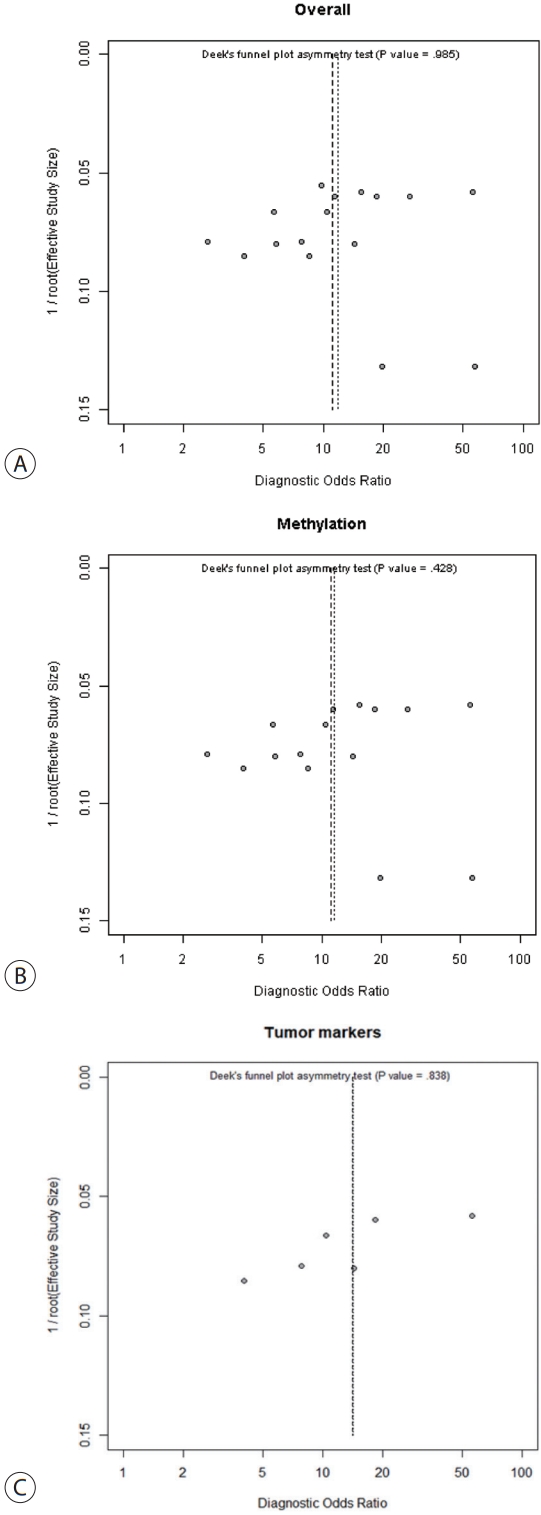
The TP, TN, FP, and FN data were extracted from all the studies. Heterogeneity was assessed by calculating the I2 statistic. An I2 value >50% reflected significant heterogeneity, and a random-effects model was then used for the analysis. Threshold effects, regression, and subgroup analyses were used to identify sources of heterogeneity. We derived the pooled SEN, SPE, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), summary receiver operating characteristic curve (SROC), and area under the curve (AUC) for all subjects and subgroups. The Fagan Nomogram was used to validate clinical utility and the Deek funnel plot asymmetry test was used to detect publication bias (P<0.10 indicated such bias). We utilized the Rex software (version 3.6.0, Rex Soft Inc., Seoul, Korea) for analysis.
RESULTS
1. Study characteristics
The PRISMA flow diagram is shown in Fig. 1. We initially retrieved 303 publications, but ultimately included nine studies19,31-38 from 2013 to 2019 after excluding duplicates and studies that were poorly designed, and carefully reviewed the titles, abstracts, and full texts. The included studies enrolled 1,042 HCC patients and 1,119 controls. Most of the controls had benign liver disease (chronic hepatitis B or cirrhosis), but 149 of them were healthy. Table 1 summarizes the significant features of these studies. Of the nine studies, seven explored the diagnostic utility of ctDNA methylation32-38 and two explored the utility of other genetic variants in HCC ctDNA.19,31 Seven articles assessed the diagnostic utility of a combination of ctDNA and tumor marker assays.19,32-37 In the six studies that reported sampling times, five samples were collected before treatment, and one sample was collected before diagnosis. ctDNA was obtained from the plasma (n=2) or serum (n=7). The assay methods used included methylation-specific polymerase chain reaction and bisulfite sequencing.
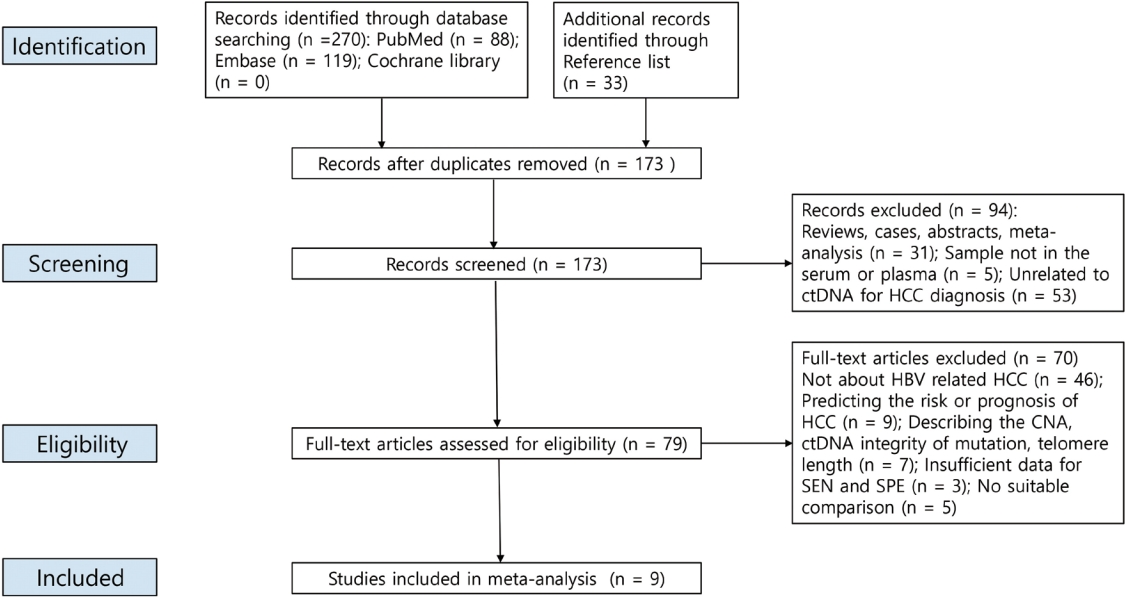
A PRISMA flow diagram of the literature search. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; ctDNA, circulating tumor DNA; Hcc, hepatocellular carcinoma; HbV, hepatitis b virus; cNA, copy number aberration; SEN, sensitivity; SPE, specificity.
2. Quality assessment
The quality of the nine studies is shown in Fig. 2 and Supplementary Table 2. Most were of moderate-to-high quality. However, four patients were at risk of patient selection bias because consecutive or random sampling was absent, the study design was not case-controlled, or patients were inappropriately excluded. Four studies may be associated with an unknown risk of bias in terms of the index test; it is unclear if the test results were interpreted without knowledge of the reference data or whether the threshold was predefined. All the reference standards exhibited a low risk of bias.
3. Diagnostic value of ctDNA assay in HBVinduced HCC patients
The SENs and SPEs of various ctDNAs in the peripheral blood of patients with HBV-induced HCC were evaluated using forest plots (Fig. 3A). Significant heterogeneity was evident in terms of both SENs (I2=91.1%, P<0.001) and SPEs (I2=92.0%, P<0.001); the SENs and false-positive rates were positively correlated (rho 0.273; 95% confidence interval [CI], -0.239 to 0.667). The meta-analysis employed a random-effects model. The pooled sensitivity and specificity were 0.705 (95% CI, 0.629-0.771) and 0.833 (95% CI, 0.769-0.882), respectively. The DOR, PLR, and NLR were 11.759 (95% CI, 7.982-17.322), 4.285 (95% CI, 3.098-5.925), and 0.366 (95% CI, 0.301-0.336), respectively, and the SROC curve AUC was 0.835 (Fig. 4A).
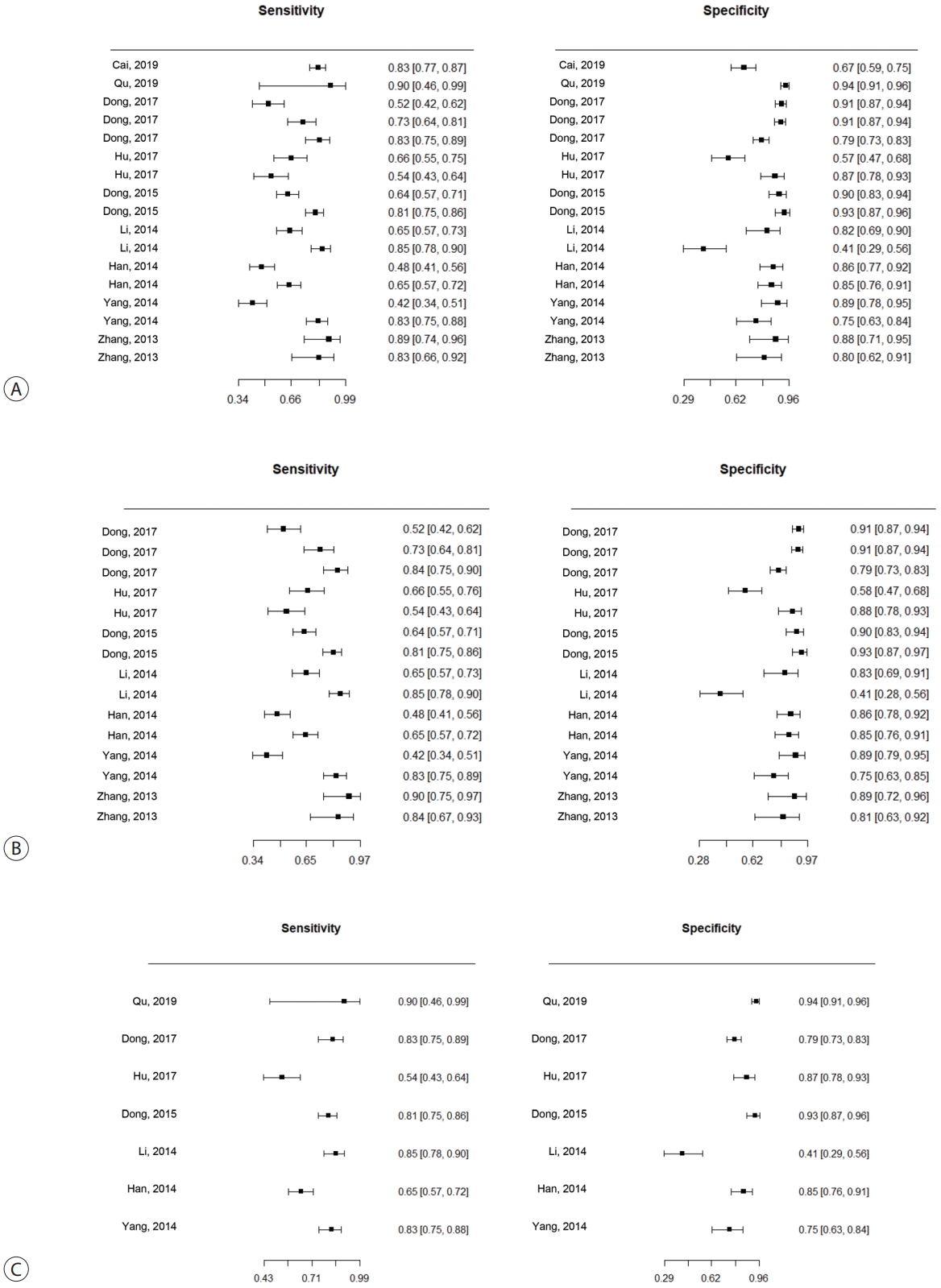
Forest plots of sensitivity and specificity of ctDNA assay for diagnosing HbV-related Hcc. (A) Overall ctDNA assay. (b) ctDNA methylation. (c) ctDNA assay combined with tumor markers. ctDNA, circulating tumor DNA; HbV, hepatitis b virus; Hcc, hepatocellular carcinoma.
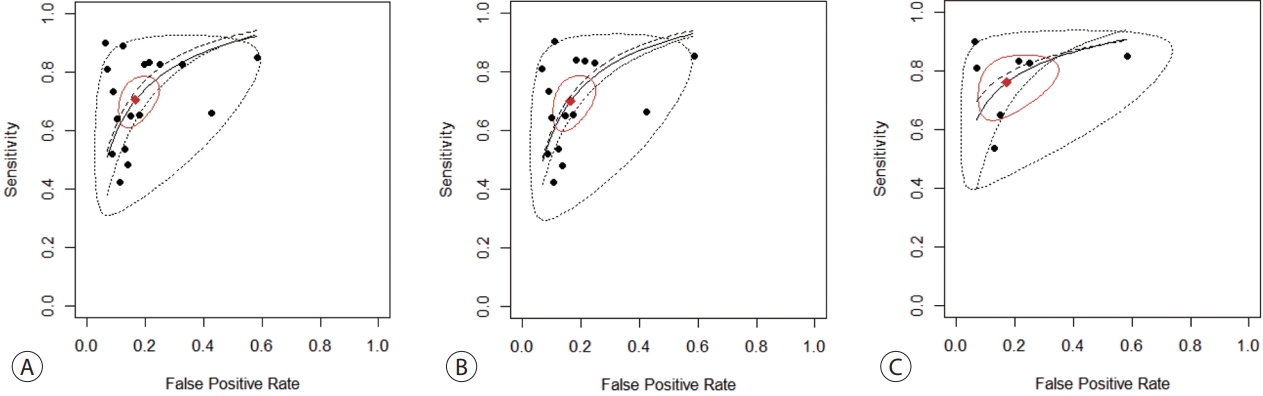
SROc curves of diagnostic value for ctDNA assay for diagnosing HbV-related Hcc. (A) Overall ctDNA assay. (b) ctDNA methylation. (c) ctDNA assay combined with tumor markers. SROc, summary receiver operating characteristic; ctDNA, circulating tumor DNA; HbV, hepatitis b virus; Hcc, hepatocellular carcinoma.
4. Diagnostic value of ctDNA methylation assay in patients with HBV-induced HCC
Of the nine studies, seven explored the diagnostic utility of ctDNA methylation assays. Significant heterogeneity was evident in terms of SENs (I2=89.7%, P<0.01) and SPEs (I2=89.1%, P<0.001). The pooled SEN and SPE were 0.7 (95% CI, 0.61-0.771) and 0.835 (95% CI, 0.768-0.885), respectively (Fig. 3B). The DOR, PLR, and NLR were 11.524 (95% CI, 7.539-17.616), 4.102 (95% CI, 2.925-5.752), and 0.379 (95% CI, 0.310-0.463), respectively. The SROC curve exhibited an AUC of 0.836 (Fig. 4B).
5. Diagnostic value of the ctDNA assay combined with tumor markers in patients with HBV-induced HCC
Seven studies explored the diagnostic utility of combined ctDNA and tumor marker assays. One study measured both α-fetoprotein (AFP) and des-gamma-carboxy-prothrombin (DCP) levels, while the others considered only AFP levels. The I2 values were 66.9% for SENs and 85.0% for SPEs. The pooled SEN and SPE were 0.760 (95% CI, 0.659-0.839) and 0.828 (95% CI, 0.692-0.911), respectively (Fig. 3C), and the AUC of the SROC curve was 0.848 (Fig. 4C). The DOR, PLR, and NLR values were 14.273 (95% CI, 7.299-27.910), 4.641 (95% CI, 2.563-8.402), and 0.304 (95% CI, 0.215-0.430), respectively.
6. Meta-regression analysis and publication bias
Meta-regression analysis was performed to explore the potential causes of heterogeneity. Sample sources, assay methods, number of genes studied, and inclusion of tumor markers did not significantly contribute to heterogeneity (Table 2). Publication bias was assessed using the Deek funnel plot asymmetry test. No significant overall bias was evident (P=0.985) (Fig. 5A), and no bias was apparent in either the ctDNA methylation group (P=0.428) (Fig. 5B), or the ctDNA with tumor markers group (P=0.838) (Fig. 5C).
DISCUSSION
HBV infection remains the principal risk factor for HCC and, thereby, for cancer-associated deaths. One of the factors primarily responsible for the poor prognosis of HCC is its low early diagnosis rate. Despite various surveillance programs, many patients are still diagnosed at advanced stages, when optimal treatment is often impossible. It is important to detect HCC at an early stage to increase the survival rate of patients.
With the increasing advances in sequencing technology, novel tools for diagnosing liver diseases are emerging. Liquid biopsy is attracting attention as a noninvasive and reliable biomarker. Circulating extracellular vesicles, DNA, RNA, and tumor cells have emerged as attractive liquid biopsy candidates because they fulfil the key requirements of ideal biomarkers.39 The application of novel molecular techniques to liquid biopsies has improved our understanding of the impact of ctDNA detection on HCC diagnosis.40 We conducted a systematic meta-analysis of studies on ctDNA-based HCC diagnosis, particularly in patients with chronic hepatitis B.
In this meta-analysis, various ctDNA assays, including somatic mutations and methylation, had a fair diagnostic performance for HBV-related HCC with an AUC of 0.835. The ctDNA methylation assay group showed a similar performance, with an AUC of 0.836. Notably, when ctDNA assays were combined with assays of tumor markers such as AFP and DCP, the diagnostic performance improved (AUCs of 0.838 and 0.829 with and without tumor marker assays, respectively; data not shown) in terms of discriminating HCC patients from control individuals. Previous studies have demonstrated that AFP exhibits unsatisfactory diagnostic performance (SENs, 0.478-0.540).41,42 When combined, detection of ctDNA and AFP assay showed an excellent diagnostic performance (AUC, 0.944).42 Along with our results, these results highlight that combinations of ctDNA and tumor markers have the potential to be novel adjuvant noninvasive tools for tumor markers in the screening and detection of HCC, regardless of its etiology.
We measured the DORs, wherein a DOR >10 indicated that the test discrimination was satisfactory.43 The pooled DOR for the ctDNA assay that discriminated HCC patients from controls was 11.759, but this increased to 14.273 when the assay was combined with tumor markers, indicating the capability of integrating ctDNA assay with tumor markers to screen and detect HCC. The PLR and NLR values were also derived. The PLRs of the ctDNA assay alone and in combination with tumor marker assays were 4.285 and 4.641, respectively, indicating that HCC cases were 4- to 5-fold more likely to be ctDNA-positive than controls. The NLRs of the ctDNA assay alone and in combination with tumor marker assays were 0.366 and 0.304, respectively, indicating that the probability that individuals who tested negative in the ctDNA assay would in fact have HCC was 36.6% and 30.4%, respectively. Therefore, the negative ctDNA assay results should be interpreted with caution.
This study had several limitations. First, despite a thorough literature search, several valuable articles might not have been included by the search strategy. Although we added these articles manually as much as possible, there is still the possibility that suitable publications were not included. Second, a significant among-study heterogeneity was observed. We performed a meta-regression to explore the potential causes of heterogeneity, but the sample source, assay method, number of genes studied, and selected tumor markers did not explain the heterogeneity. Heterogeneity might have arisen for other reasons that could not be assessed in this study because of a partial lack of data. Lastly, there are no data comparing the accuracy of ctDNA with surveillance versus surveillance alone for early HCC diagnosis. Further largescale prospective clinical studies are needed to confirm the diagnostic utility of ctDNA assays in HCC. In conclusion, ctDNA assays may aid in HCC screening and detection, particularly when combined with tumor marker assays.
Notes
Conflicts of Interest
Young Chang, Hyuksoo Eun and Do Seon Song currently serve as editorial board members for J Liver Cancer, and they were not involved in the review process of this article. Otherwise, the authors have no conflicts of interest to disclose.
Ethics Statement
This systemic review and meta-analysis is fully based on the articles which was already published and did not involve additional patient participants. Therefore, IRB approval is not necessary.
Funding Statement
This study was supported by the Soonchunhyang University Research Fund and the National Research Foundation of Korea grant funded by the Korea government (2020R1F1A1076282, 2021R1I1A3059993). This study was supported by the Research Award of the Korean Liver Cancer Association (2020).
Data Availability
The data presented in this study are available upon reasonable request from the corresponding author.
Author Contribution
Study concept and design: JSW, CY, PS
Acquisition of data: EH, SDS, LYS, CY, JSW
Analysis and interpretation of data: LHW, KSG, LSH, KW, YSJ, JJY, RS, CY, JSW
Drafting of the manuscript: CY, JSW
Statistical analysis: PS, CY, JSW
Study supervision: JSW, CY
Writing review & editing: all authors
Approval of final manuscript: all authors
Supplementary Material
Supplementary data can be found with this article online https://doi.org/10.17998/jlc.2022.09.19.