Articles
- Page Path
- HOME > J Liver Cancer > Volume 24(1); 2024 > Article
-
Original Article
Additional nodules detected using EOB-MRI in patients with resectable single hepatocellular carcinoma: an implication for active treatment strategy -
Na Reum Kim1*
 , Seoung Yoon Rho2*
, Seoung Yoon Rho2* , Jonathan Navarro3*
, Jonathan Navarro3* , Chansik An4
, Chansik An4 , Dai Hoon Han1
, Dai Hoon Han1 , Jin Sub Choi1
, Jin Sub Choi1 , Myeong-Jin Kim5†
, Myeong-Jin Kim5† , Gi Hong Choi1†
, Gi Hong Choi1†
-
Journal of Liver Cancer 2024;24(1):92-101.
DOI: https://doi.org/10.17998/jlc.2024.01.25
Published online: February 14, 2024
1Division of Hepatobiliary & Pancreatic Surgery, Department of Surgery, Yonsei University College of Medicine, Seoul, Korea
2Department of Surgery, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea
3Division of Surgical Oncology, Department of Surgery, Vicente Sotto Memorial Medical Center, Cebu, Philippines
4Department of Radiology, CHA University Bundang Medical Center, Seongnam, Korea
5Department of Radiology, Yonsei University College of Medicine, Seoul, Korea
- Corresponding author: Myeong Jin Kim, Department of Radiology, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea E-mail: kimmex@yuhs.ac
- Corresponding author: Gi Hong Choi, Division of Hepatobiliary & Pancreatic Surgery, Department of Surgery, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea E-mail: CHOIGH@yuhs.ac
- *These three authors contributed equally to this work.
†These two authors contributed equally as corresponding author to this work.
© 2024 The Korean Liver Cancer Association.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 775 Views
- 42 Downloads
Abstract
-
Background/Aim
- Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (EOBMRI) further enhances the identification of additional hepatic nodules compared with computed tomography (CT) alone; however, the optimal treatment for such additional nodules remains unclear. We investigated the long-term oncological effect of aggressive treatment strategies for additional lesions identified using EOB-MRI in patients with hepatocellular carcinoma (HCC).
-
Methods
- Data from 522 patients diagnosed with solitary HCC using CT between January 2008 and December 2012 were retrospectively reviewed. Propensity score-matched (PSM) analysis was used to compare the oncologic outcomes between patients with solitary HCC and those with additional nodules on EOB-MRI after aggressive treatment (resection or radiofrequency ablation [RFA]).
-
Results
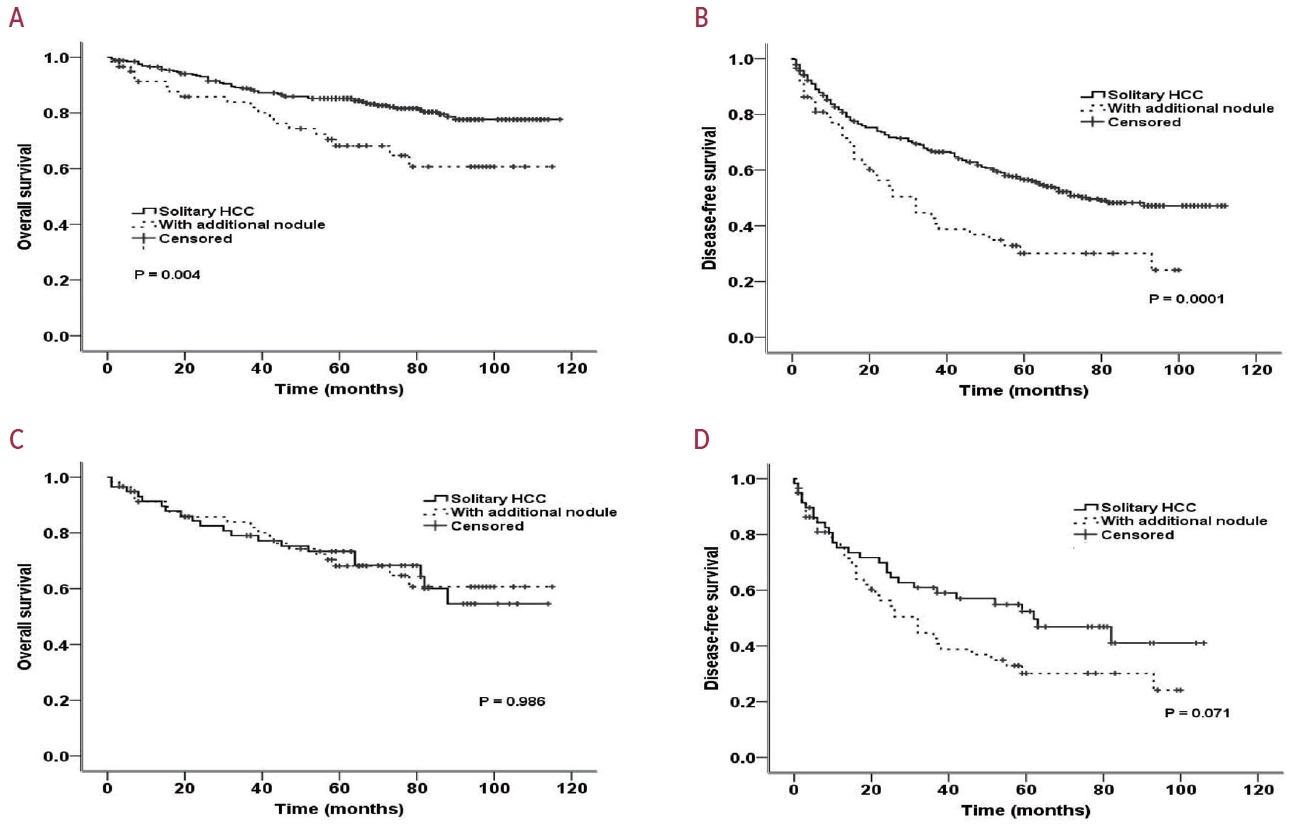
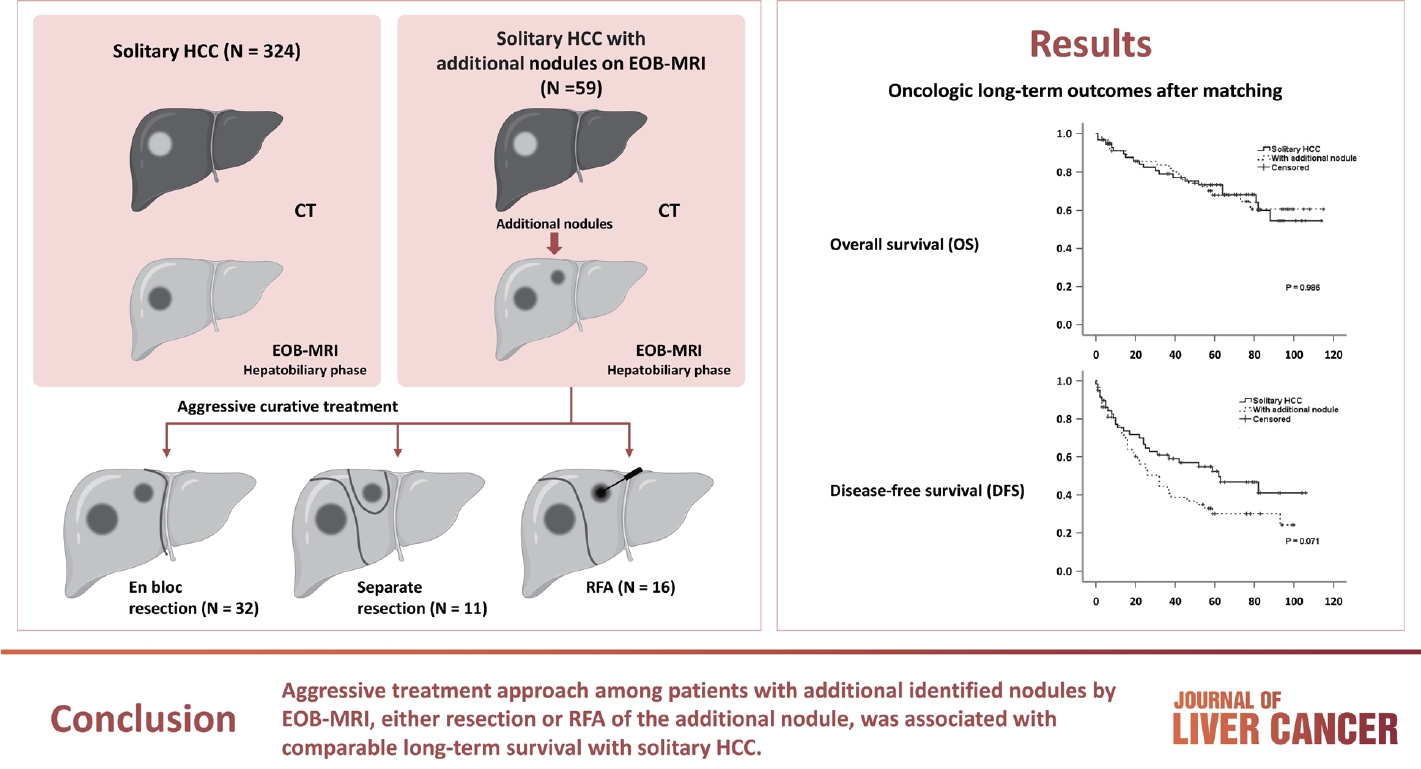
- Among the 383 patients included, 59 had additional nodules identified using EOB-MRI. Compared with patients with solitary HCC, those with additional nodules on EOB-MRI had elevated total bilirubin, aspartate transaminase, and alanine transaminase; had a lower platelet count, higher MELD score, and highly associated with liver cirrhosis (P<0.05). Regarding long-term outcomes, 59 patients with solitary HCC and those with additional nodules after PSM were compared. Disease-free survival (DFS) and overall survival (OS) were comparable between the two groups (DFS, 60.4 vs. 44.3 months, P=0.071; OS, 82.8 vs. 84.8 months, P=0.986).
-
Conclusion
- The aggressive treatment approach, either resection or RFA, for patients with additional nodules identified on EOBMRI was associated with long-term survival comparable with that for solitary HCC. However, further studies are required to confirm these findings.
- Despite the evolving treatment modalities for hepatocellular carcinoma (HCC) in the past decades,1 it remains one of the most common causes of cancer-related mortality worldwide.2 Although the treatment paradigm varies substantially across countries, early detection remains the most effective approach to improve the survival outcome of patients with HCC.3 The emerging role of new sophisticated diagnostic modalities, such as multidetector computed tomography (CT) scan and gadolinium‐ethoxybenzyl‐diethylenetriamine pentaacetic acid‐enhanced magnetic resonance imaging (EOB-MRI), further enhanced the detection of early HCC.4 Consensus was reached by the Korean Society of abdominal radiology in Korea on the feasibility of EOB-MRI as one of the diagnostic tools for the evaluation and recognition of early HCC.5
- As multicentricity is one of the pathological highlights of HCC,6,7 the diseased liver may warrant a thorough evaluation for proper staging and prognostication. The pivotal role of EOB-MRI in identifying additional nodules superseded that of the multidetector CT scan capability.8,9 Preoperative concurrent non-hypervascular hypointense hepatic nodules detected using EOB-MRI in patients with HCC were associated with a high incidence rate of recurrence.10,11 Moreover, additional EOB-MRI pretreatment evaluation in patients with single HCC led to the detection of additional nodules and a better recurrence-free and overall survival after treatment, attributed to the avoidance of futile surgical resection and better surgical candidate selection.12 Nevertheless, the optimal management, whether combined curative treatment or other non-surgical treatments, for such an additional lesion identified using EOB-MRI in patients with resectable HCC remains controversial. Therefore, we aimed to investigate the long-term survival outcomes of an aggressive treatment strategy for additional lesions identified using EOB-MRI in patients with HCC.
INTRODUCTION
- Patients, study design, and follow-up
- The medical records of 522 patients with HCC between January 2008 and December 2012 were retrospectively reviewed from the electronic medical records of Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. Patients with a history of interventional therapy for HCC, such as radiofrequency ablation (RFA) or transcatheter arterial chemoembolization (TACE) (n=53), the presence of multiple tumors (n=40), previous history of CT scan (n=64), association with other malignancies (n=7), or missing data (n=2) were excluded, finally including 383 consecutive patients. Among them, 324 were confirmed to have a single HCC lesion on both CT and EOB-MRI, whereas 59 had additional nodules on EOB-MRI. This study was approved by the Yonsei Institutional Review Board (IRB), and informed consent was waived because of the retrospective nature of the study (IRB No. 4-2018-0650).
- In this cohort, our active treatment strategy included resection (anatomical or non-anatomical) or intraoperative RFA of the additionally identified nodule on EOB-MRI, as appropriate, based on tumor location and liver function. Serum alphafetoprotein (AFP), prothrombin induced by vitamin K absence/antagonist-II (PIVKA-II) and radiological monitoring were performed every three months during the follow-up visit. Recurrence was defined as any suspected lesion on imaging, with or without elevated serum AFP or PIVKA-II levels. Treatment strategies for recurrence include RFA, TACE, or liver transplantation, as appropriate.
- Preoperative imaging evaluation of the liver nodule
- A multidetector computed tomography (MDCT) scan (Somatom sensation 64; Siemens Medical Solution, Forchhein, Germany) and an MRI 3.0 system (Magnetom Trio Tim; Siemens Medical Solution) were used at our center. MDCT images were obtained during the pre-contrast, arterial, portal, venous, and equilibrium phases. The EOB-MRI used the volume-interpolated breath-hold examination sequence, and images were obtained in a transverse plane using a 12-channel coil before (pre-contrast), at 20 seconds (arterial phase), 60 seconds (portal venous phase), 180 seconds (late dynamic phase), and 20 minutes (hepatobiliary phase) after administering the contrast agent. Using a power injector, 0.025 mmol/kg body weight of gadoxetate disodium (Primovist; Bayer Schering Pharma, Berlin, Germany) was injected via a peripheral venous access catheter (cubital vein or cephalic vein access) at a rate of 2 mL/s, which was subsequently flushed with 20 mL saline at the same rate.
- The MDCT and EOB-MRI scans were interpreted by three radiologists with substantial expertise in liver imaging. The preoperative diagnosis of HCC was based on the European Association for the Study of the Liver (EASL) panel of experts and the American Association for the Study of Liver Diseases (AASLD) radiologic hallmark of HCC: the contrast uptake in the arterial phase (arterial enhancement) and washout in the venous/late phase.13,14 Additional nodules included 1) nodules not identified by preoperative MDCT scan and 2) hypervascular or non-hypervascular nodules, exhibiting hypointensity during the hepatobiliary phases of EOB-MRI.
- Statistical analysis
- Data analyses were performed using IBM SPSS Statistics version 22 (SPSS Inc., Chicago, IL, USA). Categorical variables were expressed as frequencies (%), whereas continuous variables were presented as means with their range or ± standard deviation, as appropriate. Overall survival (OS) and disease-free survival (DFS) were estimated using the Kaplan-Meier method. The log-rank test was used to determine risk factors associated with OS and DFS. Statistical significance was defined as a P<0.05.
- Propensity score matching (PSM) was used to reduce the confounding effects on preoperative baseline and clinical factors between patients with solitary HCC and those with additional nodules. The matching factors included age, sex, body mass index (BMI), platelet count, liver enzymes (aspartate transaminase [AST] and alanine transaminase [ALT]), prothrombin time (INR), total bilirubin level, model for end-stage liver disease (MELDs) score, AFP, PIVKA-II, tumor size, presence of cirrhosis, microvascular invasion, tumor grade, and gross tumor morphology. Propensity scores were estimated using logistic regression analysis, and matching was performed using the nearest neighbor method with a 0.2-width caliper at a 1:1 ratio.
METHODS
- Patient characteristics
- Table 1 summarizes the clinical characteristics of patients with solitary HCC and those with additional nodules identified on EOB-MRI. The mean age was 55.64±9.95 years. Three hundred two (78.8%) were men, and 81 (21.1%) were women. At a median follow-up of 71 months (range, 3-117), 78 (20.4%) died, whereas 190 (49.6%) experienced tumor recurrence.
- Compared with patients with solitary HCC, those with additional nodules on EOB-MRI had elevated serum total bilirubin, ALT, and AST; had a lower platelet count, higher MELD score, highly associated with liver cirrhosis (P<0.05) (Table 1).
- Table 2 summarizes the types of hepatectomies performed for solitary HCC and describes the treatment strategies for additional nodules in detail. Regarding treatment strategy, anatomical resection was commonly performed to treat the solitary HCC and HCC with additional nodule groups (83.0% and 86.4%, respectively). Because half of the additional nodules were located in the same lobe of the liver (n=32, 54.2%), en bloc resection of the additional nodules was performed in 32 of 59 patients (eight sectionectomies, 15 right hemihepa-tectomies, seven left hemihepa-tectomies, and two central bisectionectomies). Additional resections (non-anatomical) were performed in 11 of the 27 patients with additional nodules located away from the primary tumor, whereas 16 patients underwent RFA. EOB-MRI changed the initial surgical plan (extent of resection [n=16], addition of wedge resection [n=11], and intraoperative RFA [n=16]) in 43 (11.2%) of 383 patients with only MDCT. The types of hepatectomies performed for solitary HCC and solitary HCC with additional nodules were not significantly different (Table 2). Concerning short-term outcomes, complications occurred in 35 (10.8%) and 10 (6.9%) patients, respectively, with no significant difference (P=0.177). Similarly, major complications were observed in 24 (7.4%) and six (10.2%) patients, respectively, without a significant difference (P=0.435).
- Table 3 presents characteristics of EOB-MRI for additional nodules and compares two groups: one with pathologically confirmed HCC and the other without. The mean size of additional nodules is 1.0±0.4 cm, ranging from 0.4-2.0 cm. Overall, 26 patients underwent resection for additional nodules and were pathologically confirmed to have HCC. Among the 33 patients, six underwent resection but were pathologically confirmed to have lesions other than HCC (two necrotic lesions, one hemorrhagic nodule, two dysplastic nodules, and one von Meyenburg complex). Eleven patients underwent en bloc resection of the main lesion. However, no additional nodules were detected or evaluated pathologically. Sixteen patients underwent RFA but were unable to undergo pathological evaluation for additional nodules. Arterial enhancement was significantly higher in the pathologically confirmed HCC group than in the other without (65.4% vs. 27.3%; P=0.003). Washout in the portal venous phase did not differ between groups. High signal intensity on T2 images and diffusion-weighted images was higher in the group with pathologic confirmation of HCC than in the group without pathologic confirmation of HCC (69.2% vs. 36.4%; P=0.012 and 45.8% vs. 16.1%; P=0.016, respectively).
- Long-term survival outcome analysis
- PSM analysis was performed to obtain similar clinical characteristics between the two groups (solitary HCC vs. HCC with additional nodules) (Table 4). Fig. 1A and B show the OS and DFS rates of the two groups before and after PSM, respectively. After PSM, although the DFS remained poor among patients with an additionally identified nodule on EOB-MRI (Fig. 1C), the difference was not statistically significant (60.4 vs. 44.3 months; P=0.071). No significant difference in OS between patients with solitary HCC and those with additional nodules (82.8 vs. 84.8 months, respectively; P=0.986) was observed (Fig. 1D).
RESULTS
- Despite the increasing understanding of various treatment modalities for HCC, early diagnosis and treatment remains one of the most effective management methods to improve its oncologic outcome and prognosis.4 Henceforth, the dramatic acceptance of the emerging role of EOB-MRI for early detection of HCC can be discerned from its innate superiority compared with the multidetector CT scan in terms of sensitivity and specificity in the HCC diagnosis.15 In particular, relatively strong pieces of evidence were extrapolated for the efficacy of EOB-MRI over MDCT in patients with small HCC <2 cm and cirrhotic liver.9,15,16
- Given the relatively high predisposition of HCC to multifocal disease, either multicentric or intrahepatic metastasis,17,18 the relevance of EOB-MRI in detecting additional nodules compared with MDCT has been demonstrated in various studies.19-21 Recently, Kim et al.22 reported that the application of gadoxetic acid-enhanced MRI enabled the identification of 57 additional nodules in 53 (16.4%) of 323 patients who underwent CT and EOB-MRI. These additional nodules were actively treated simultaneously or within 3 months of the primary treatment. Additional evaluation using EOB-MRI was an independent prognostic factor for HCC recurrence and overall mortality after treatment. Concerning the main reasons for these improved long-term outcomes, rather than actively treating these additional HCCs, they attributed this improved outcome to the avoidance of futile surgical resection and modification of appropriate treatment plans because 53 patients with additional HCC nodules received surgical resection less frequently than that of the 270 patients who had no additional HCC nodules (47.2 vs. 70.0%; P=0.001).22 In cases wherein additional HCC nodules were actively treated, they failed to demonstrate the effect of active treatment on long-term outcomes because the liver condition and primary treatment of the main tumor were heterogeneous. Therefore, we focused on patients with Child-Pugh class A (well-preserved) liver function who tolerated some extent of liver resection to investigate the efficacy of combined active curative treatments of additional nodules on the long-term outcomes.
- Several studies have investigated the influence of hypovascular hypointense nodules on long-term prognosis in patients with overt HCC using EOB-MRI. Toyoda et al.10 reported that the presence of untreated hypovascular nodules among patients with HCC who underwent hepatectomy was associated with a higher risk for multicentric recurrence compared with patients with no hypointense nodule or with hypointense nodule included in hepatectomy, suggesting that the presence of such nodules may indicate an increase in hepatocarcinogenesis. A recent meta-analysis also reported that the hypervascular transformation of hypovascular hypointense nodules in the hepatobiliary phase of EOB-MRI was 30% within 3 years, and initial nodular size (cut-off value, 9 mm) was a significant factor for malignant transformation.23 In our study, patients with additional nodules had higher levels of liver enzymes and bilirubin, lower platelet count, and more cirrhotic liver than patients with no additional nodules, all these suggesting a higher potential of multicentric carcinogenesis in the background liver.
- Although immediate surgical resection is not recommended for patients with primary hypovascular hypointense nodules on EOB-MRI,24 aggressive surgical approaches involving resection and RFA may have some advantages in patients with overt HCC and additional hypointense nodules and is the most effective curative treatment for patients with overt HCC. For additional nodules, curative treatments, including resection or RFA, can be applied at one stage, and additional treatment can be avoided during the follow-up period because a significant proportion of additional nodules transform into overt HCC, and curative treatments are usually limited to patients with recurrent HCC who underwent previous surgical resections. Our results showed that 11% of patients have nodules additionally identified using EOB-MRI, which cannot be observed by MDCT alone. Our results suggest that these groups of patients showed no significant differences in terms of long-term survival (Fig. 1). As such, even though the presence of a multifocal tumor in patients with HCC was associated with a higher rate of tumor recurrence and poor OS,25,26 our preoperative early detection of multifocal HCC enabled the modification and optimization of our treatment approach in 43 (12.1%) of 354 patients, either en bloc resection, separate resections, or RFA of the additional tumor as appropriate, resulting in comparable DFS and OS compared with patients with single HCC and no additional nodules after PSM analysis.22,27
- EOB-MRI in this cohort enabled the identification of additional hepatic nodules with the potential to progress to overt HCC, mostly less than 1 cm in size. Retrospective data have shown that an acceptable long-term survival outcome can be achieved for selected patients with multiple HCCs, especially for three or fewer tumors with a <3 cm diameter.28,29 Hence, this study further supports the oncological benefit of resection or ablation for additional nodules detected using EOB-MRI or active treatment of selected patients with multiple HCC. Although this active treatment approach may not translate to improved overall survival outcomes in this cohort, the presence of multifocal HCC provided a substantial opportunity to actively follow up with these patients and treat any tumor recurrence whenever necessary. As a result, an improvement in overall survival can still be attained through early detection and appropriate management of tumor recurrence.30,31
- This study had several limitations. There is no data on the exclusion of initial surgical candidates due to the detection of additional nodules on EOB-MRI. However, our study suggests that patients with acceptable liver function should be considered for surgery. Furthermore, we did not evaluate the long-term outcomes in patients with additional nodules who were under observation without aggressive treatment. This limitation prevents us from directly confirming the clinical benefits of aggressive treatment in patients with additional nodules. Therefore, further comparative analysis of patients with solitary HCC and those with additional nodules, considering whether they received aggressive treatment, is necessary to obtain more robust evidence. The control group, with a single HCC and no additional nodules, had different hepatitis activities and fibrosis stages. Although a propensity score analysis was performed to eliminate potential bias between the two groups, the relatively small sample size may hinder the generalization of our results. Therefore, a multicenter study is warranted.
- In summary, EOB-MRI enables the identification of additional nodules that cannot be identified using MDCT alone. With an aggressive treatment approach, the long-term survival is similar to that of patients with solitary HCC. However, further studies are required to confirm these findings.
DISCUSSION
-
Conflict of Interest
The authors have no conflicts of interest to disclose.
-
Ethics Statement
This study was approved by the Yonsei Institutional Review Board, and informed consent was waived because of the retrospective nature of the study (IRB No. 4-2018-0650).
-
Funding Statement
This study was supported by the Korean Liver Cancer Association Research Award (2022).
-
Data Availability
The data presented in this study are available upon request from the corresponding author.
-
Author Contribution
Conceptualization: SYR, JN, GHC
Data curation: SYR, JN, CA, DHH, JSC, MJK
Formal analysis: JN
Supervision: GHC
Writing - original draft: SYR, NRK, JN, GHC
Writing - review & editing: SYR, NRK, JN, CA, MJK, GHC
All authors approved the final version of the manuscript.
Article information
Values are presented as mean±standard deviation or number (%).
HCC, hepatocellular carcinoma; EOB-MRI, gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging; BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; NBNC, non-B, non-C hepatitis; INR, international normalized ratio; MELD, model for endstage liver disease; AST, aspartate transaminase; ALT, alanine transaminase; AFP, alpha-fetoprotein; PIVKA-II, prothrombin-induced by vitamin K absence or antagonist-II.
| Type of hepatectomy | Solitary HCC (n=324) | Solitary HCC + additional nodule (n=59) | P-value |
|---|---|---|---|
| Before matching | |||
| Wedge | 55 (17.0) | 8 (13.6) | 0.426 |
| Segmentectomy | 66 (20.4) | 7 (11.9) | |
| Sectionectomy | 57 (17.6) | 16 (27.1) | |
| Hemihepatectomy | 120 (37) | 24 (40.7) | |
| Central hepatectomy | 26 (8.0) | 4 (6.8) | |
| After matching* | |||
| Wedge | 10 (16.0) | 8 (13.6) | 0.900 |
| Segmentectomy | 9 (15.3) | 7 (11.9) | |
| Sectionectomy | 14 (23.7) | 16 (27.1) | |
| Hemihepatectomy | 23 (39.0) | 24 (40.7) | |
| Central hepatectomy | 3 (5.1) | 4 (6.8) | |
| Treatment for additional nodule | |||
| En bloc resection with primary lesion (anatomical) | 32 (54.2) | ||
| Sectionectomy | 8 (13.6) | ||
| Right hemihepatectomy | 15 (25.4) | ||
| Left hemihepatectomy | 7 (11.9) | ||
| Central bisectionectomy | 2 (3.4) | ||
| Separate (non-anatomical) resection for additional nodules | 11 (18.6) | ||
| RFA for additional nodules | 16 (27.1) | ||
| Non-anatomical resection + RFA | 2 (3.4) | ||
| Anatomical resection + RFA | 14 (23.7) |
| EOB-MRI findings for additional nodules | Total (n=59) | Pathology-confirmed HCC (n=26) | Non pathology-confirmed HCC (n=33)* | P-value |
|---|---|---|---|---|
| Tumor size on MRI (cm) | 1.0±0.4 (0.4-2.0) | 0.9±0.3 (0.4-1.5) | 1.0±0.3 (0.5-2.0) | 0.063 |
| Arterial enhancement | ||||
| No | 33 (55.9) | 9 (34.6) | 24 (72.7) | 0.003 |
| Yes | 26 (44.1) | 17 (65.4) | 9 (27.3) | |
| Wash out in portal venous phase | ||||
| No | 24 (40.7) | 8 (30.8) | 16 (48.5) | 0.169 |
| Yes | 35 (59.3) | 18 (69.2) | 17 (51.5) | |
| High signal in T2 image | ||||
| No | 29 (49.2) | 8 (30.8) | 21 (63.6) | 0.012 |
| Yes | 30 (50.8) | 18 (69.2) | 12 (36.4) | |
| High signal in diffusion weighted image† | ||||
| No | 39 (66.1) | 13 (54.2) | 26 (83.9) | 0.016 |
| Yes | 16 (27.1) | 11 (45.8) | 5 (16.1) |
Values are presented as mean±standard deviation (range) or number (%).
EOB-MRI, gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging; HCC, hepatocellular carcinoma.
* The non pathology-confirmed HCC group included 17 patients who underwent resection, without pathology evaluation or with pathology indicating diagnoses other than HCC, and 16 patients who underwent radiofrequency ablation without pathology evaluation;
† Except 4 patients have no diffusion weighted image.
Values are presented as mean±standard deviation or number (%).
HCC, hepatocellular carcinoma; EOB-MRI, gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging; BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; NBNC, non-B, non-C hepatitis; INR, international normalized ratio; MELD, model for endstage liver disease; AST, aspartate transaminase; ALT, alanine transaminase; AFP, alpha-fetoprotein; PIVKA-II, prothrombin-induced by vitamin K absence or antagonist-II.
- 1. Wang CH, Wey KC, Mo LR, Chang KK, Lin RC, Kuo JJ. Current trends and recent advances in diagnosis, therapy, and prevention of hepatocellular carcinoma. Asian Pac J Cancer Prev 2015;16:3595−3604.ArticlePubMed
- 2. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589−604.ArticlePubMedPMCPDF
- 3. Kudo M. Management of hepatocellular carcinoma in Japan as a worldleading model. Liver Cancer 2018;7:134−147.ArticlePubMedPMCPDF
- 4. Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, et al. Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res 2019;49:1109−1113.ArticlePubMedPDF
- 5. Korean Liver Cancer Association (KLCA), National Cancer Center (NCC). 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol 2019;20:1042−1113.ArticlePubMedPMCPDF
- 6. Okusaka T, Okada S, Ueno H, Ikeda M, Shimada K, Yamamoto J, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer 2002;95:1931−1937.ArticlePubMed
- 7. Schlageter M, Terracciano LM, D’Angelo S, Sorrentino P. Histopathology of hepatocellular carcinoma. World J Gastroenterol 2014;20:15955−15964.ArticlePubMedPMC
- 8. Golfieri R, Garzillo G, Ascanio S, Renzulli M. Focal lesions in the cirrhotic liver: their pivotal role in gadoxetic acid-enhanced MRI and recognition by the Western guidelines. Dig Dis 2014;32:696−704.ArticlePubMedPDF
- 9. Liu X, Jiang H, Chen J, Zhou Y, Huang Z, Song B. Gadoxetic acid disodium-enhanced magnetic resonance imaging outperformed multidetector computed tomography in diagnosing small hepatocellular carcinoma: a meta-analysis. Liver Transpl 2017;23:1505−1518.ArticlePubMedPDF
- 10. Toyoda H, Kumada T, Tada T, Niinomi T, Ito T, Sone Y, et al. Non-hypervascular hypointense nodules detected by Gd-EOB-DTPA-enhanced MRI are a risk factor for recurrence of HCC after hepatectomy. J Hepatol 2013;58:1174−1180.ArticlePubMed
- 11. Iwamoto T, Imai Y, Igura T, Kogita S, Sawai Y, Fukuda K, et al. Nonhypervascular hypointense hepatic nodules during the hepatobiliary phase of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced MRI as a risk factor of intrahepatic distant recurrence after radiofrequency ablation of hepatocellular carcinoma. Dig Dis 2017;35:574−582.ArticlePubMedPDF
- 12. Shim JH, Han S, Shin YM, Lee YJ, Lee SG, Kim KM, et al. Prognostic performance of preoperative gadoxetic acid-enhanced MRI in resectable hepatocellular carcinoma. J Magn Reson Imaging 2015;41:1115−1123.ArticlePubMedPDF
- 13. European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908−943.ArticlePubMed
- 14. Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005;42:1208−1236.ArticlePubMed
- 15. Li J, Wang J, Lei L, Yuan G, He S. The diagnostic performance of gadoxetic acid disodium-enhanced magnetic resonance imaging and contrastenhanced multi-detector computed tomography in detecting hepatocellular carcinoma: a meta-analysis of eight prospective studies. Eur Radiol 2019;29:6519−6528.ArticlePubMedPDF
- 16. Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology 2015;275:97−109.ArticlePubMed
- 17. Feo F, Pascale RM. Multifocal hepatocellular carcinoma: intrahepatic metastasis or multicentric carcinogenesis? Ann Transl Med 2015;3:4. PubMedPMC
- 18. Yang SL, Luo YY, Chen M, Zhou YP, Lu FR, Deng DF, et al. A systematic review and meta-analysis comparing the prognosis of multicentric occurrence and vs. intrahepatic metastasis in patients with recurrent hepatocellular carcinoma after hepatectomy. HPB (Oxford) 2017;19:835−842.ArticlePubMed
- 19. Hammerstingl R, Huppertz A, Breuer J, Balzer T, Blakeborough A, Carter R, et al. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol 2008;18:457−467.ArticlePubMedPDF
- 20. Ichikawa T, Saito K, Yoshioka N, Tanimoto A, Gokan T, Takehara Y, et al. Detection and characterization of focal liver lesions: a Japanese phase III, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease. Invest Radiol 2010;45:133−141.PubMed
- 21. Ye F, Liu J, Ouyang H. Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging and multidetector-row computed tomography for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e1157.PubMedPMC
- 22. Kim HD, Lim YS, Han S, An J, Kim GA, Kim SY, et al. Evaluation of earlystage hepatocellular carcinoma by magnetic resonance imaging with gadoxetic acid detects additional lesions and increases overall survival. Gastroenterology 2015;148:1371−1382.ArticlePubMed
- 23. Suh CH, Kim KW, Pyo J, Lee J, Kim SY, Park SH. Hypervascular transformation of hypovascular hypointense nodules in the hepatobiliary phase of gadoxetic acid-enhanced MRI: a systematic review and meta-analysis. AJR Am J Roentgenol 2017;209:781−789.ArticlePubMed
- 24. Lee DH, Lee JM, Yu MH, Hur BY, Yi NJ, Lee KW, et al. Non-hypervascular hepatobiliary phase hypointense nodules on gadoxetic acidenhanced MR can help determine the treatment method for HCC. Eur Radiol 2019;29:3122−3131.ArticlePubMedPDF
- 25. Hao S, Fan P, Chen S, Tu C, Wan C. Distinct recurrence risk factors for intrahepatic metastasis and multicenter occurrence after surgery in patients with hepatocellular carcinoma. J Gastrointest Surg 2017;21:312−320.ArticlePubMedPDF
- 26. Toyama T, Hiramatsu N, Yakushijin T, Oze T, Nakanishi F, Yasumaru M, et al. A new prognostic system for hepatocellular carcinoma including recurrent cases: a study of 861 patients in a single institution. J Clin Gastroenterol 2008;42:317−322.PubMed
- 27. Choi SH, Choi GH, Kim SU, Park JY, Joo DJ, Ju MK, et al. Role of surgical resection for multiple hepatocellular carcinomas. World J Gastroenterol 2013;19:366−374.ArticlePubMedPMC
- 28. Yamakado K, Nakatsuka A, Takaki H, Yokoi H, Usui M, Sakurai H, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology 2008;247:260−266.ArticlePubMed
- 29. Glantzounis GK, Paliouras A, Stylianidi MC, Milionis H, Tzimas P, Roukos D, et al. The role of liver resection in the management of intermediate and advanced stage hepatocellular carcinoma. A systematic review. Eur J Surg Oncol 2018;44:195−208.ArticlePubMed
- 30. Zhang X, Li C, Wen T, Yan L, Li B, Yang J, et al. Appropriate treatment strategies for intrahepatic recurrence after curative resection of hepatocellular carcinoma initially within the Milan criteria: according to the recurrence pattern. Eur J Gastroenterol Hepatol 2015;27:933−940.PubMed
- 31. Koh PS, Chan AC, Cheung TT, Chok KS, Dai WC, Poon RT, et al. Efficacy of radiofrequency ablation compared with transarterial chemoembolization for the treatment of recurrent hepatocellular carcinoma: a comparative survival analysis. HPB (Oxford) 2016;18:72−78.ArticlePubMedPMC
References
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation
- Download Citation
- Close
- Related articles
-
- The diagnostic value of circulating tumor DNA in hepatitis B virus induced hepatocellular carcinoma: a systematic review and meta-analysis
- Stereotactic body radiation therapy for elderly patients with small hepatocellular carcinoma: a retrospective observational study
- Effect of direct-acting antivirals for hepatitis C virus-related hepatocellular carcinoma recurrence and death after curative treatment

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION


 Follow JLC on Twitter
Follow JLC on Twitter