Articles
- Page Path
- HOME > J Liver Cancer > Volume 23(2); 2023 > Article
-
Original Article
Clinical outcome of surgical resection for multifocal T2-T3 hepatocellular carcinoma up to 3 nodules: a comparative analysis with a single nodule -
Sehyeon Yu
 , Hye-Sung Jo
, Hye-Sung Jo , Young-Dong Yu
, Young-Dong Yu , Yoo jin Choi
, Yoo jin Choi , Dong-Sik Kim
, Dong-Sik Kim
-
Journal of Liver Cancer 2023;23(2):377-388.
DOI: https://doi.org/10.17998/jlc.2023.08.24
Published online: September 15, 2023
Division of HBP Surgery and Liver Transplantation, Department of Surgery, Korea University College of Medicine, Seoul, Korea
-
Corresponding author: Dong-Sik Kim, Division of HBP Surgery and Liver Transplantation, Department of Surgery, Korea University College of Medicine, 73 Goryeodae-ro, Seongbuk-gu, Seoul 02841, Korea
Tel. +82-2-920-6620, Fax. +82-2-921-6620 E-mail: kimds1@korea.ac.kr
© 2023 The Korean Liver Cancer Association.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 661 Views
- 37 Downloads
Abstract
-
Background/Aims
- Although the Barcelona Clinic Liver Cancer staging system seems to underestimate the impact of curative-intent surgical resection for multifocal hepatocellular carcinoma (HCC), recent studies have indicated favorable results for the surgical resection of multiple HCC. This study aimed to assess clinical outcomes and feasibility of surgical resection for multifocal HCC with up to three nodules compared with single tumor cases.
-
Methods
- Patients who underwent surgical resection for HCC with up to three nodules between 2009 and 2020 were included, and those with the American Joint Committee on Cancer (AJCC) 8th edition, T1 and T4 stages were excluded to reduce differences in disease distribution and severity. Finally, 81 and 52 patients were included in the single and multiple treatment groups, respectively. Short- and long-term outcomes including recurrence-free survival (RFS) and overall survival (OS), were evaluated.
-
Results
- All patients were classified as Child-Pugh class A. RFS and OS were not significantly different between the two groups (P=0.176 and P=0.966, respectively). Multivariate analysis revealed that transfusion and intrahepatic metastasis were significantly associated with recurrence (P=0.046 and P=0.005, respectively). Additionally, intrahepatic metastasis was significantly associated with OS (hazard ratio, 1.989; 95% confidence interval, 1.040-3.802; P=0.038).
-
Conclusions
- Since there was no significant difference in survival between the single and multiple groups among patients with AJCC 8th stage T2 and T3, surgical resection with curative intent could be considered with acceptable long-term survival for selected patients with multiple HCC of up to three nodules.
- Primary liver cancer is the sixth most prevalent and second leading cause of cancer-related deaths in the Republic of Korea.1 Hepatocellular carcinoma (HCC), constituting the majority of primary liver cancers, shows different prognoses depending on the degree of cancer progression and residual liver function. The number of HCC nodules is related to recurrence and intrahepatic metastasis, which are risk factors associated with a poor prognosis.2-4
- The Barcelona Clinic Liver Cancer (BCLC) staging system is widely used to assess the severity of HCC and guide treatment decisions considering factors such as tumor size, number of tumors, liver function, presence of vascular invasion, and extrahepatic spread.5,6 Factoring the portal pressure and bilirubin level, surgical liver resection is recommended as the first treatment option for single-nodule HCC only. However, in the recently updated BCLC guideline, non-surgical treatments, such as radiofrequency ablation (RFA), liver transplantation (LT), trans-arterial chemoembolization (TACE), and systemic treatment, are recommended as the first-line treatment options in the case of multinodular HCCs.7 Although LT is regarded optimal for treatment outcomes and recommended for patients with multinodular HCCs within the Milan criteria, their feasibility is hindered by the limited availability of liver grafts.8
- However, several recent studies have reported that liver resection, as an initial treatment for multinodular HCC up to three, showed favorable outcomes compared to RFA or TACE regarding survival rate.9-16 The BCLC staging system may limit the availability of curative-intent surgical liver resection for multinodular HCCs. The 2022 hepatocellular carcinoma treatment guidelines of the Korean Liver Cancer Association (KLCA)-National Cancer Center (NCC) Korea state that surgical resection may be considered in patients with three or fewer multiple HCCs and well-preserved liver function (grading of recommendations, assessment, development, and evaluation low-weak [C2]).17 Given that tumor multiplicity is a negative prognostic factor, we included only patients with T2 or T3 stage and excluded patients with T1 stage, who were dominant in the single group and were expected to have a relatively favorable prognosis. Finally, this study aimed to identify the clinical outcomes of surgical resection for multifocal HCC of up to three nodules by comparing them with those obtained from single tumor resection. Furthermore, we aimed to verify contradictory outcomes regarding the role of liver resection in managing multiple HCCs.
INTRODUCTION
- 1. Patients’ selection and study design
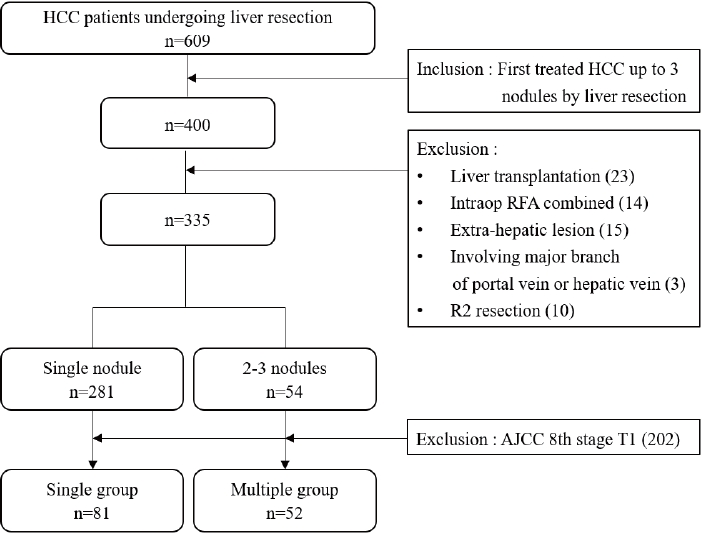
- Patients with up to three nodules who underwent liver resection as the first treatment between 2009 and 2020 were included in this study. The following were excluded: (1) LT, (2) combined intraoperative ablation therapy, (3) extrahepatic metastasis, (4) major vascular invasion, and (5) R2 resection. The 335 patients were divided into two groups according to the number of tumors identified using imaging studies; 281 and 54 patients had single nodules and 2-3 nodules, respectively. Among the patients with a single nodule in the imaging study, 200 (60.3%) were of the American Joint Committee on Cancer (AJCC) 8th T1 stage. In contrast, only two (3.8%) of the 2-3 nodules were in the T1 stage. T1 stage cases were excluded to reduce the disease distribution and severity differences. Finally, AJCC 8th edition T2 and T3 patients were selected, and the outcomes of liver resection were compared between the single (n=81) and multiple (n=52) groups (Fig. 1).
- 2. Preoperative evaluation
- All patients were evaluated using three-phase computed tomography (CT) and magnetic resonance imaging (MRI) with liver-specific contrast to confirm the HCC diagnosis, tumor number, size, vascular invasion, and underlying liver cirrhosis. HCC was diagnosed if the imaging findings were consistent with those of HCC, at a size larger than or equal to 1 cm, hyper-enhancement in the arterial phase, and washout in the portal venous or delayed phase on imaging studies in high-risk patients. Resectability was evaluated by comprehensively considering the tumor status and underlying liver function. The indocyanine green retention rate at 15 minutes was evaluated to assess the liver functional reserve.
- 3. Outcomes and follow-up
- All postoperative complications were prospectively recorded and stratified according to the Clavien-Dindo classification. Post-hepatectomy liver failure was determined according to the definitions of the International Study Group of Liver Surgery. Clinical follow-ups included physical examinations and laboratory tests with CT performed 4 weeks after surgery and every 3-6 months subsequently. Overall survival (OS) was defined as the time from surgery to death or last follow-up. Recurrence-free survival (RFS) was defined as the time from surgery to the first locoregional or systemic recurrence or the last follow-up.
- 4. Statistical analysis
- Continuous variables were presented as medians and ranges, and categorical variables as numbers and percentages. Continuous variables were compared between groups using Student’s t-test and Mann-Whitney U test. Categorical variables were compared using the chi-square or Fisher’s exact test, as appropriate. OS and RFS rates were calculated using Kaplan-Meier analysis and compared using log-rank tests. Statistical significance was set at P<0.05. IBM SPSS Statistics for Windows (version 20.0; IBM Corp., Armonk, NY, USA) was used for all the statistical analyses.
METHODS
- 1. Baseline characteristics
- The baseline patient characteristics are shown in Table 1. Regarding underlying liver disease, the proportions of patients with hepatitis B virus, hepatitis C virus, and alcoholic liver disease were comparable between the single and multiple groups (P=0.209, P=0.339, and P=0.209, respectively). The total bilirubin and platelet counts were not significantly different between the single and multiple groups (1.0 [0.29-1.56] vs. 1.0 [0.25-1.54] mg/dL; P=0.234 and 176,000 [33,000-593,000] vs. 162,500 [75,000-419,000]; P=0.189, respectively). All patients in this study satisfied Child-Pugh class A criteria, and the median model for end-stage liver disease score was comparable between the groups (7 [6-21] vs. 7 [6-12]; P=0.949). Maximum tumor size was larger in the single group than in the multiple group (6 [1.2-22] vs. 4 [1.2-16]; P=0.035).
- 2. Pathologic examination and short-term outcome
- Pathological examination results after liver resection are shown in Table 2. Since the single and multiple groups were classified according to the radiological results, the tumor number on pathological examination was not the same as in preoperative imaging studies. Nine (11.1%) and two (3.8%) patients were in the single and multiple groups, respectively. Major and worst tumors were not differentiated between the groups (P=0.120 and P=0.769, respectively). Although R1 resection (microscopically margin-positive) was observed only in the single group (n=4, 4.9%), the difference was not statistically significant (P=0.155). Microvascular invasion was more prevalent in the single group than in the multiple group (67 [82.7%] vs. 25 [48.1%]; P<0.001). Because this study classified single and multiple groups based on radiological results (CT and MRI) and included only patients in T2 or T3 stages based on pathology, care should be taken when interpreting the results.
- In terms of short-term outcomes in Table 3, the laparoscopic approach was performed more frequently in the single group than in the multiple group (16 [19.8%] vs. 2 [3.8%]; P=0.009). The number of patients who received transfusions was comparable between the two groups (14 [17.3%] vs. 10 [19.2%]; P=0.776). Complications of Clavien–Dindo grade IIIA or above occurred in 14 patients (17.3%) in the single group and in five patients (9.6%) in the multiple group (P=0.217). The length of hospital stay did not differ between the groups (11 [5-72] vs. 11 [7-33], P=0.749).
- The major hepatectomy (hemi-hepatectomy and extended hemi-hepatectomy) was performed at similar rates in both groups (21 [25.9%] vs. 13 [25.0%]; P=0.905). Among them, there were five (four and one in each group, P=0.364) major complications of Clavien–Dindo grade IIIA or above. Complications included respiratory failure, intra-abdominal fluid collection, postoperative bleeding, bile leakage, and wound infection. However, the 30-day and 90-day mortality rates were not observed in either group.
- 3. Long-term outcome
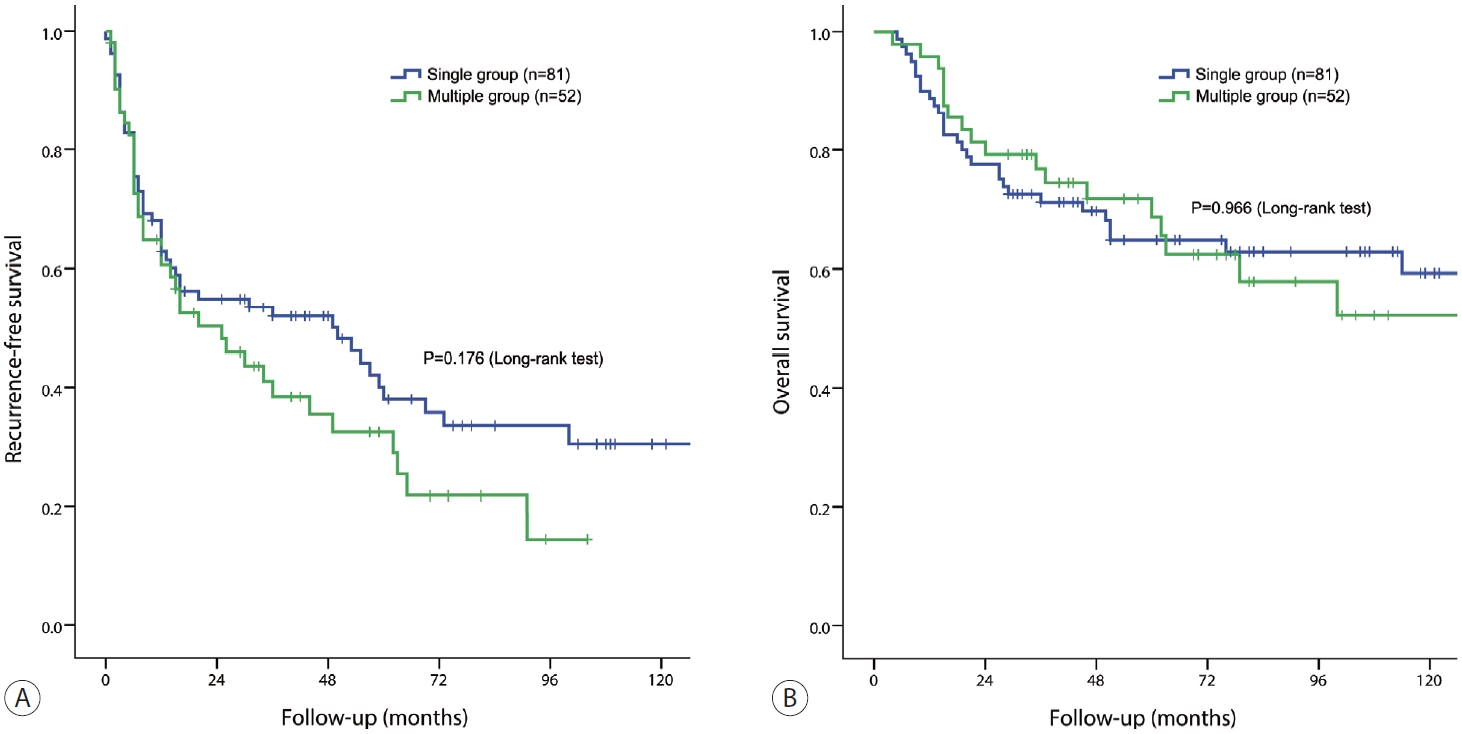
- The 1-, 3-, and 5-year RFS rates in the single group were 62.8%, 54.9%, and 38.1%, respectively, whereas those in the multiple groups were 60.7%, 50.3%, and 32.5%, respectively (P=0.176) (Fig. 2A). The 1-, 3-, and 5-year OS rates of the single group were 88.8%, 77.5%, and 64.8%, respectively, whereas those of the multiple groups were 95.8%, 79.2%, and 68.8%, respectively (P=0.966) (Fig. 2B).
- Multivariate analysis showed that the tumor number was not a significant risk factor for RFS or OS in this cohort (Table 4). Intrahepatic metastasis was an independent risk factor for both RFS and OS (hazard ratio [HR], 2.064; 95% confidence interval [CI], 1.249-3.411; P=0.005 and HR, 1.989; 95% CI, 1.040-3.802; P=0.038, respectively). In addition, transfusion was a risk factor for RFS (HR, 1.772; 95% CI, 1.009-3.112; P=0.046).
- We also determined RFS and OS by classifying patients into single and multiple groups based on pathology. The study included 72 and 57 patients, respectively. Similar to the previous results of the radiology-based group classification, no significant difference in RFS and OS was found between the two groups (P=0.200 and P=0.862, respectively) (Fig. 3).
- 4. Post-recurrence treatment
- Among the 85 patients with recurrent HCC in this study, 49 were in the single HCC group (60.4%), and 36 were in the multiple HCC group (69.2%). Several therapies were administered in combination to these patients, including surgical liver resection, TACE, RFA, and radiotherapy (15, 31, 18, and seven patients in the single HCC group vs. 16, 27, 16, and five patients in the multiple HCC group, respectively). Additionally, systemic treatments such as sorafenib (12 and five patients, respectively), regorafenib (three patients in the single HCC group), and lenvatinib (three and one patient, respectively) were used for palliative treatment.
- Of the 31 patients who underwent surgery after recurrence, 19 underwent additional hepatectomy and two underwent adrenalectomy. Metastatic lymph node excision or metastasectomy was performed in three patients, and wedge resection of the lung due to lung metastasis was performed in two patients. Five patients underwent living donor liver transplantation.
RESULTS
- This study aimed to compare the clinical outcomes of surgical liver resection between single and multifocal HCCs with ≤3 nodules. Previous studies comparing hepatic resection with TACE in the intermediate stage reported that OS after surgical resection was significantly better than that in the TACE groups (23.9-60% vs. 12-41.6%).14-16 In addition, recent studies have also shown that liver resection has a favorable survival rate compared to non-surgical treatment in multinodular HCCs with ≤3 nodules.4,14,18
- BCLC staging, which is widely used to assess HCC and determine its first treatment option, recommends nonsurgical treatment (such as RFA, TACE, and systemic treatment) or liver transplantation for multinodular HCC. For multifocal HCC within the Milan criteria (BCLC stage A), liver resection is assumed to increase the recurrence risk.19-23 As prospective clinical research to define when liver resection should be given priority over TACE or ablation is lacking, whether liver resection could be applied as the first treatment for multifocal HCC within the Milan criteria remains debatable. Patients with intermediate (BCLC stage B) HCC are a heterogeneous population because of the variability in tumor size, number, and liver function. Although there are other methods, such as Bolondi’s subclassification, to distinguish the BCLC stage B, the BCLC 2022 guidelines state that it is difficult to make robust cut-offs for the BCLC stage B. Therefore, recent attempts have been made to subgroup patients with BCLC stage B to confirm the feasibility of surgical resection.24 According to the results of this study, no significant difference in RFS and OS in liver resection between single HCC and multifocal HCCs (up to three) in AJCC 8th T2 and T3 existed. Considering these results, whether a single tumor is a prerequisite for curative-intent liver resection is questionable.
- In the first stage of patient classification, imaging rather than pathological results were used. Based on the pathological examination results, there were cases of multiple HCCs in the single group and vice versa. This is because the information we have at the time of deciding on surgery is imaging results, such as CT or MRI. We believed that the number of HCCs observed using tools that could be evaluated at the time of diagnosis was important; therefore, we classified the groups based on imaging studies. Multiple HCC (2-5 nodules) were identified in 11.1% of the patients in the single group on pathological examination. Most of these were cases with additional HCC (<0.5 cm) insensitive to imaging.
- Patients at both extremes (AJCC 8th edition T1 and T4) were excluded from the study to reduce the difference in HCC severity between single and multiple groups. Patients with a single nodule of less than 2 cm or major vessel invasion were excluded; therefore, they were considered a subgroup of BCLC stages A and B. As we excluded T1 patients expected to have a favorable prognosis, the single group showed a higher proportion of microvascular invasion according to the AJCC 8th edition staging system. This might be a limitation due to biased patient selection; however, it should be emphasized that there were no significant differences in long-term survival between the single and multiple groups in the selected patients in this study.
- However, considering that OS was higher than RFS (38.1% and 32.5% vs. 64.8% and 68.6%, respectively), it is imperative to determine the treatments administered after recurrence. TACE and RFA are the most frequently used post-recurrence treatments. Surgical resection, including liver transplantation, was performed after HCC recurrence in some patients (15 and 16 cases, respectively). Many patients with recurrences receive multiple treatments.
- In the multivariate analysis, the factors associated with RFS were blood transfusion and intrahepatic metastasis. Blood transfusion increases the recurrence rate because of immune dysfunction.25,26 One hypothesis is that some important immune cells and their functions, such as cytotoxic mediators, natural killer cell activity, and lymphocyte count, are decreased after transfusion of allogeneic red blood cells, resulting in a reduction of cytotoxic cell activity and inhibition of interleukin-2 production. Suppressor T-cell activity and immunosuppressive prostaglandins are increased.26 Whether these changes actually promote the recurrence of HCC is still debatable.27,28 However, many studies on colorectal cancers have reported a similar result that immune dysfunction after transfusion of red blood cells is associated with cancer spread.29,30 Prospective studies and considerations of the degree and timing of blood transfusion and the freshness of blood products are needed.
- Intrahepatic metastasis, a well-known poor prognostic factor, was the only factor associated with OS in the present study. As intrahepatic metastasis was present in 30.8% of patients in the single group and 19.2% in the multiple group (P=0.13), the number of HCC did not appear to be strongly associated with intrahepatic metastasis. Intrahepatic metastasis of HCC is related to underlying liver cirrhosis, tumor characteristics, vascular invasion, and immunosuppression. In previous studies, coagulation and fibrinolytic factors may have regulated the degree of intrahepatic metastasis by conferring anoikic resistance to HCC cells and promoting HCC cell escape from immune attacks.31
- As a single-center retrospective study, this study had some limitations, such as selection bias, small sample size, and short median follow-up period. As many surgeons refer to imaging studies when determining the initial treatment for HCC, the use of imaging studies to classify single and multiple groups had the advantage of similarity to the actual medical field. However, this may have influenced the differences in characteristics between the two groups. Regarding patient selection, since the single group had both the positive factor of solitary nodules and the negative factor of microvascular invasion, this could be a confounding limitation. However, the long-term survival was comparable between the single and multiple groups according to the selection criteria used in this study. Finally, nonsurgical treatment outcomes were not directly compared in this study.
- In conclusion, since there was no significant difference in survival between the single and multiple groups among AJCC 8th stage T2 and T3, surgical resection with curative intent could be considered with acceptable long-term survival for selected patients with multiple HCC of up to three nodules.
DISCUSSION
-
Conflict of Interest
The authors have no conflicts of interest to disclose.
-
Ethics Statement
This study was approved by the Institutional Review Board of Korea University Anam Hospital (2019AN0219) and the need for informed consent was waived due to the retrospective nature of this study.
-
Funding Statement
This study was supported by the Korean Liver Cancer Association Research Award (2021).
-
Data Availability
The data presented in this study are available upon request from the corresponding author.
-
Author Contribution
Conceptualization: SY, DSK
Data curation: SY, HSJ, YDY, YJC
Formal analysis: SY, YDY, YJC
Investigation: SY, YDY, YJC
Methodology: SY, YDY, YJC
Project administration: HSJ, DSK
Resources: SY, YDY, YJC
Software: SY, YDY, YJC
Supervision: HSJ, DSK
Validation: HSJ, DSK
Visualization: SY, YDY, YJC
Writing–original draft: SY, DSK
Writing–review & editing: SY, HSJ, YDY, YJC, DSK
Approval of the final version of the manuscript: all authors
Article information
Acknowledgments
Supplementary Material

Values are presented as median (range) for continuous data and number (%) for categorical data.
BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; PT, prothrombin time; ICG, indocyanine green; MELD, model for end-stage liver disease; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence-II; BCLC, Barcelona Clinic Liver Cancer.
- 1. Park HM, Won YJ, Kang MJ, Park SJ, Kim SW, Jung KW, et al. Trend analysis and prediction of hepatobiliary pancreatic cancer incidence and mortality in Korea. J Korean Med Sci 2022;37:e216.ArticlePubMedPMCPDF
- 2. Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology 2008;134:1908−1916.ArticlePubMed
- 3. Ho MC, Huang GT, Tsang YM, Lee PH, Chen DS, Sheu JC, et al. Liver resection improves the survival of patients with multiple hepatocellular carcinomas. Ann Surg Oncol 2009;16:848−855.ArticlePubMedPDF
- 4. Fukami Y, Kaneoka Y, Maeda A, Kumada T, Tanaka J, Akita T, et al. Liver resection for multiple hepatocellular carcinomas: a Japanese nationwide survey. Ann Surg 2020;272:145−154.PubMed
- 5. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 2016;150:835−853.ArticlePubMed
- 6. Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329−338.ArticlePubMed
- 7. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, GarciaCriado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 2022;76:681−693.ArticlePubMedPMC
- 8. Varma V, Mehta N, Kumaran V, Nundy S. Indications and contraindications for liver transplantation. Int J Hepatol 2011;2011:121862. ArticlePubMedPMC
- 9. Kang TW, Kim JM, Rhim H, Lee MW, Kim YS, Lim HK, et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection--propensity score analyses of long-term outcomes. Radiology 2015;275:908−919.ArticlePubMed
- 10. Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg 2014;260:329−340.ArticlePubMed
- 11. Hyun MH, Lee YS, Kim JH, Lee CU, Jung YK, Seo YS, et al. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: a meta-analysis of highquality studies. Hepatology 2018;68:977−993.ArticlePubMedPDF
- 12. Liu PH, Hsu CY, Hsia CY, Lee YH, Huang YH, Chiou YY, et al. Surgical resection versus radiofrequency ablation for single hepatocellular carcinoma ≤ 2 cm in a propensity score model. Ann Surg 2016;263:538−545.ArticlePubMed
- 13. Vitale A, Burra P, Frigo AC, Trevisani F, Farinati F, Spolverato G, et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol 2015;62:617−624.ArticlePubMed
- 14. Lu L, Zheng P, Wu Z, Chen X. Hepatic resection versus transarterial chemoembolization for intermediate-stage hepatocellular carcinoma: a cohort study. Front Oncol 2021;11:618937. ArticlePubMedPMC
- 15. Zhang DZ, Wei XD, Wang XP. Comparison of hepatic resection and transarterial chemoembolization for solitary hepatocellular carcinoma. World J Gastroenterol 2015;21:4635−4643.ArticlePubMedPMC
- 16. Cassese G, Han HS, Cho JY, Lee HW, Lee B, Troisi RI. Selecting the best approach for the treatment of multiple non-metastatic hepatocellular carcinoma. Cancers (Basel) 2022;14:5997. ArticlePubMedPMC
- 17. Korean Liver Cancer Association (KLCA), National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. J Liver Cancer 2023;23:1−120.ArticlePubMedPMCPDF
- 18. Yue YY, Zhou WL. Hepatic resection is associated with improved long-term survival compared to radio-frequency ablation in patients with multifocal hepatocellular carcinoma. Front Oncol 2020;10:110. ArticlePubMedPMC
- 19. Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol 2013;59:89−97.ArticlePubMed
- 20. N’Kontchou G, Mahamoudi A, Aout M, Ganne-Carrié N, Grando V, Coderc E, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 2009;50:1475−1483.ArticlePubMed
- 21. Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol 2012;107:569−577. quiz 578.ArticlePubMedPMCPDF
- 22. Cucchetti A, Djulbegovic B, Tsalatsanis A, Vitale A, Hozo I, Piscaglia F, et al. When to perform hepatic resection for intermediatestage hepatocellular carcinoma. Hepatology 2015;61:905−914.ArticlePubMedPDF
- 23. Forner A, Gilabert M, Bruix J, Raoul JL. Reply: heterogeneity of intermediate-stage HCC necessitates personalized management including surgery. Nat Rev Clin Oncol 2015;12:10. ArticlePDF
- 24. Zhaohui Z, Shunli S, Bin C, Shaoqiang L, Yunpeng H, Ming K, et al. Hepatic resection provides survival benefit for selected intermediate-stage (BCLC-B) hepatocellular carcinoma patients. Cancer Res Treat 2019;51:65−72.ArticlePubMedPMCPDF
- 25. Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, et al. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery 1994;115:303−309.PubMed
- 26. Harada N, Shirabe K, Maeda T, Kayashima H, Ishida T, Maehara Y. Blood transfusion is associated with recurrence of hepatocellular carcinoma after hepatectomy in Child-Pugh class A patients. World J Surg 2015;39:1044−1051.ArticlePubMedPDF
- 27. Kwon AH, Matsui Y, Kamiyama Y. Perioperative blood transfusion in hepatocellular carcinomas: influence of immunologic profile and recurrence free survival. Cancer 2001;91:771−778.ArticlePubMed
- 28. Ydy LR, Slhessarenko N, de Aguilar-Nascimento JE. Effect of perioperative allogeneic red blood cell transfusion on the immuneinflammatory response after colorectal cancer resection. World J Surg 2007;31:2044−2051.ArticlePubMedPDF
- 29. Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg 2012;256:235−244.PubMed
- 30. Wu HL, Tai YH, Lin SP, Chan MY, Chen HH, Chang KY. The impact of blood transfusion on recurrence and mortality following colorectal cancer resection: a propensity score analysis of 4,030 patients. Sci Rep 2018;8:13345. ArticlePubMedPMCPDF
- 31. Li X, Gu B, Wang B, Feng Z, Ma Y, Li H, et al. Intrahepatic metastases may be specific to hepatocellular carcinoma due to the coagulation and fibrinolytic systems (review). Oncol Rep 2020;44:2345−2352.ArticlePubMed
References
Figure & Data
References
Citations


 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION
 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation

 Follow JLC on Twitter
Follow JLC on Twitter