Articles
- Page Path
- HOME > J Liver Cancer > Volume 19(2); 2019 > Article
-
Case Report
Radiation-induced Myositis after Proton Beam Therapy to Huge Hepatocellular Carcinoma -
Jihye Kim1*, Gyu Sang Yoo2*, Dong Hyun Sinn1, Hee Chul Park2
 , Kwang Cheol Koh1
, Kwang Cheol Koh1 -
Journal of Liver Cancer 2019;19(2):136-142.
DOI: https://doi.org/10.17998/jlc.19.2.136
Published online: September 30, 2019
1Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
2Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- Corresponding author : Hee Chul Park Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea tel. +82-2-3410-2612, Fax. +82-2-3410-2619 e-mail; hee.ro.park@samsung.com
- *These two authors contributed equally to this work.
• Received: February 11, 2019 • Revised: March 18, 2019 • Accepted: April 3, 2019
Copyright © 2019 The Korean Liver Cancer Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 6,156 Views
- 119 Downloads
- 2 Citations
Abstract
- Proton beam therapy (PBT) is one of the advances in radiotherapy techniques, which enables dose escalation with lower probability of radiation-induced liver or gastrointestinal injuries. However, the chest wall proximal to the tumor can be affected by high dose irradiation. Here, we report on a 58-year-old male patient who presented with huge hepatocellular carcinoma, received treatment with transarterial chemoembolization and PBT, and developed severe chest wall pain due to radiation-induced myositis. The patient’s symptoms were controlled by oral steroids.
- Radiotherapy (RT) has been increasingly utilized as the local modality for hepatocellular carcinoma (HCC) [1]. Various RT techniques have been developed, that enable the delivery of high-dose radiation to the target volume accurately and safely [2]. Proton beam therapy (PBT), one of the advances in RT techniques, has dosimetric advantages over X-ray therapy due to its physical properties such as the use of the Bragg peak and the absence of an exit dose [1,3]. Therefore, PBT allows for dose escalation in the RT of HCC without increasing the probability of radiation-induced liver disease or other gastrointestinal toxicities. On the contrary, by dose escalation, the chest wall proximal to the tumor is also irradiated with high dose. Some studies have reported high rates of chest wall toxicity, such as rib fracture (16%) [4] and chest wall pain of grade 2 or more (30%) [5] after PBT for HCC. Herein, we report a case of radiation-induced myositis after PBT of HCC.
INTRODUCTION
- A 58-year-old male patient was referred to our hospital with incidentally detected huge liver mass on abdominal ultrasonography. He had a history of chronic hepatitis B virus (HBV) infection, but was not under regular HCC surveillance. The patient had no symptoms except dyspepsia. There were no remarkable findings including jaundice, palpable mass or ascites on physical examination. He had been consuming about 15 standard drinks per week for 30 years. He was a never smoker, was self-employed, and had a family history of hypertension and thyroid cancer (younger brother). The initial laboratory findings at our hospital were as follows: normal complete blood cell count with 181,000 platelets per microliter of blood; total bilirubin 0.7 mg/dL; albumin 4.3 mg/dL; aspartate aminotransferase (AST) 95 IU/L; alanine aminotransferase (ALT) 62 IU/L; alkaline phosphatase (ALP) 69 IU/L; gammaglutamyl transpeptidase (GGT) 164 IU/L; international normalized ratio (INR) 1.04; serum creatinine 1.05 mg/dL (estimated glomerular filtration rate of 78.4 mL/min/1. 73 m2). Alpha-fetoprotein (AFP) level was 17.3 mg/dL and prothrombin induced by vitamin K absence-II (PIVKA-II) level was 13,000 mAU/mL. Serum HBV DNA level was 495,573 IU/mL. The patient had good liver function with Child-Pugh class A (score 5) and the indocyanine green retention rate at 15 minutes (ICG R15) of 11.6%.
- Initial contrast-enhanced magnetic resonance images (MRI) using Primovist showed a 20 cm sized HCC with several daughter nodules in the right liver, and borderline sized lymph nodes around the hepatic artery and the portocaval space (Fig. 1). There was no distant metastasis or major vascular invasion. The clinical staging was stage III (T3N0M0) according to the modified Union for International Cancer Control (UICC) staging and stage B according to Barcelona Clinic Liver Cancer (BCLC) staging. The patient was started with antiviral treatment for HBV using entecavir, and HCC treatment using transarterial chemoembolization (TACE) followed by PBT.
- Conventional TACE was performed with an intra-arterial injection of a mixture of doxorubicin hydrochloride (Adriamycin; Dong-A Pharm, Seoul, Korea) 40 mg and iodized oil (Lipiodol; Laboratoire Andre Guerbet, Aulnay-sous-Bois, France) 10 mL after a femoral approach, celiac angiogram and superselection of tumor feeder at the level of the segmental or subsegmental artery with a micro-guidewire and a 2.0-Fr microcatheter. The feeder were embolized with gelatin sponge pledgets (Cutanplast, Mascia Brunelli S.P.A, Milano, Italy) until hemostasis was achieved.
- The day after the initial TACE, serum total bilirubin, AST, and ALT increased up to 1.7 mg/dL, 2,270 IU/L, and 484 IU/L, respectively. Serum C-reactive protein (CRP) level was elevated over 6 mg/dL and fever developed 2 days after procedure. There was no evidence of definitive infection on microbiological cultures or significant complication on immediate follow-up computed tomography (CT) scan. The patient fully recovered and was discharged after 7 days of conservative treatment under the diagnosis of post-TACE syndrome without an increase of Child-Pugh score.
- 1. Proton beam therapy
- The patient was started on PBT after three weeks from the first TACE, as scheduled. The detailed procedure of PBT in our institution has been introduced in a previous report [1]. In brief, a shallow breathing technique was chosen for the respiratory motion management of the patient. Before the simulation, training for regular and shallow breathing was performed. Respiration of the patient was guided by an upper inhalation line and an exhalation baseline set after monitoring voluntary shallow breathing. During the simulation, four-dimensional CT images encompassing the whole phase of respiration were acquired using the same devices used for respiration training (Fig. 2). The maximum intensity projection image was used as the primary image for PBT planning.
- Gross tumor volumes (GTVs) were delineated on the CT image of each respiration phase using reference contrast-enhanced MRI. Clinical target volumes (CTVs) were obtained by the expansion of GTVs by 0.5 cm. Internal target volume (ITV) was delineated by summation of all CTVs. With an additional margin of 0.5 cm from ITV, planning target volume (PTV) was defined. The treatment plan was set with a goal of delivering 72.6 cobalt gray equivalents (CGE; multiplication of proton physical dose by relative biological effectiveness of 1.1) in 22 fractions to the PTV without violating the dose constraint for normal liver (Fig. 3). The line-scanning technique was chosen for the delivery of proton beam.
- Before each treatment session, daily image guidance was performed with cone beam CT and/or orthogonal digital radiography using VeriSuite (MedCom, Darmstadt, Germany). The densely iodized oil, liver dome, and bony structure were used for tumor localization during the image guidance.
- 2. Further treatment progress
- A month after completing PBT, the patient received the second TACE. A month after the second TACE, follow-up CT images revealed the viable portion in the main hepatic mass and multiple pulmonary metastases. The patient was started with sorafenib at two months after the end of PBT. However, after two months of treatment, regorafenib replaced sorafenib because adrenal metastasis progressed.
- At 6 months after the PBT, the patient visited the emergency room presenting with severe pain on the right chest wall reaching the numeric rating scale 10. Painkillers including opioids were prescribed but were ineffective. The patient stopped taking regorafenib for a few days on his own, but felt no change in pain and resumed regorafenib. The patient had no history of trauma and skin lesions such as rash and blister on the site. He had no fever, leukocytosis, or significant changes in inflammatory markers. The chest wall between the ribs was swollen with low attenuation on emergent CT scans (Fig. 4). There was no evidence of thrombosis, abscess, or interval change in disease extent compared to the previous test.
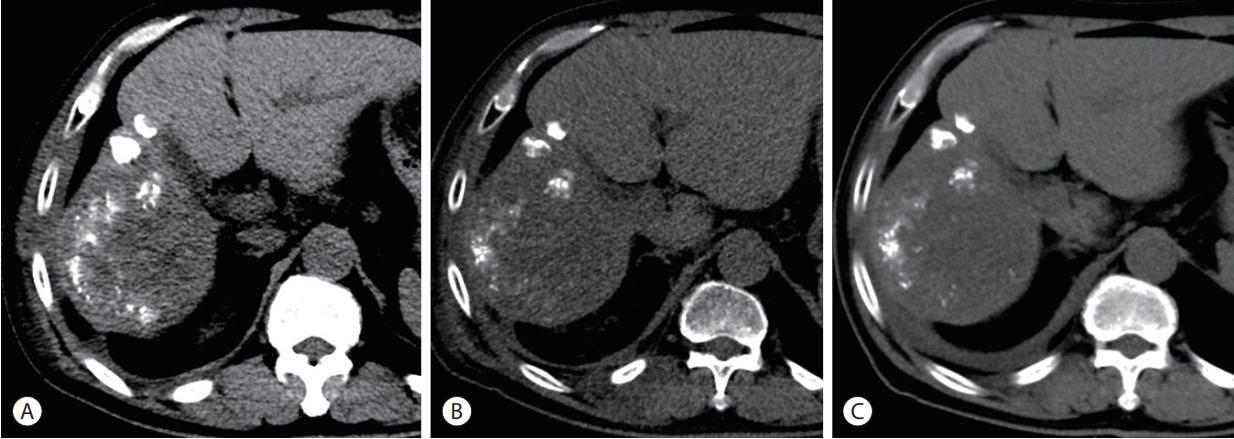
- Under the diagnosis of radiation myositis, the patient started prednisolone 30 mg once a day. On the 3rd day, the pain was greatly improved. Prednisolone was tapered off following the schedule as below; 30 mg daily for 2 weeks, 15 mg daily for 2 days, 10 mg daily for 2 days, 5 mg daily for 2 days, and was tapered off. Follow-up CT scan showed markedly decreased swelling of chest wall (Fig. 5).
- After combination of TACE and PBT treatment, intrahepatic lesions were well controlled without viable tumor for up to 10 months from HCC diagnosis, but pulmonary metastasis remained and adrenal metastasis had progressed despite three months of regorafenib therapy. Regorafenib was replaced by nivolumab, and the patient is being followed-up at the outpatient clinic. The chest wall of the PBT treatment site darkened slightly and stiffened, but the chest wall pain has not recurred up to 1 month of nivolumab treatment.
CASE REPORT
- Radiation-induced myositis is a rarely reported adverse effect of RT [6]. Histologically, radiation-induced myositis is late radiation injury to muscle, causing focal muscle degeneration, loss of capillaries and increase in collagen [7]. Radiation-induced myositis was reported as muscle injury after stereotactic body RT (SBRT) using high doses in 1 to 3 fractions. Lockney et al. [8] identified 11 (1.6%) patients showing radiographic evidence of myositis following high dose spinal SBRT among 667 patients. The median times to the development of radiographic evidence and clinical symptoms from myositis were 4.7 and 1.4 months after SBRT, respectively. On the other hand, various literatures also showed cases of myositis as radiation recall phenomenon, which is defined as acute inflammation in previously irradiated tissues, typically occurring weeks to months following RT and subsequent systemic therapy [9]. Not only cytotoxic agents such as gemcitabine, carboplatin, and paclitaxel [10,11], but also targeted agents [12,13] are known to be associated with radiation recall myositis. Mostly, radiation recall myositis was reported as case report due to its extremely low incidence [11]. In case of PBT which enables high dose irradiation on tumors without increasing the normal liver dose, the external tissue proximal to the tumor can also be irradiated with the high dose. Some literatures have reported chest wall toxicity such as rib fracture (16%) [4] and chest wall pain of grade 2 or more (30%) [5] after PBT for HCC.
- In our case, both the symptom and the radiographic finding consistent with myositis in chest wall appeared at six months after the completion of PBT. The area of myositis was the area proximal to the gross tumor volume targeted to receive the prescribed dose of 72.6 CGE in 22 fractions. Therefore, high doses of up to 71.1 CGE in 22 fractions were delivered in areas of chest wall where myositis occurred. There is no consensus regarding dose constraints for chest wall toxicity resulting from radiation myositis. However, efforts to reduce the dose delivered to the chest wall should be made. Especially, during the planning of PBT, which is more beneficial in normal liver sparing than X-ray RT, dose escalation is frequently tried. Therefore, cautions to save adjacent normal tissues reported to be not vulnerable to RT, are necessary because these normal tissues can be irradiated with high doses according to dose escalation.
- In addition to high dose irradiation to the chest wall, sorafenib or regorafenib might be trigger factors for radiation myositis due to radiation recall phenomenon. The mechanisms of radiation recall phenomenon by sorafenib are poorly understood. Phillips and Fu [14] suggested that drug sensitivity has changed by radiation, through alteration in cell age distribution. Kim et al. [15] suggested that RT induces the release of certain cytokines, including interleukin-1, interleukin-6, platelet-derived growth factor-beta, tumor necrosis factor, and transforming growth factor, which cause inflammatory response. However, the mechanisms for radiation recall are not clearly established. In addition, reports of radiation recall phenomenon by sorafenib are mainly limited to dermatitis and there is no relevant report about myositis. However, the course of myositis is similar to that in previous reports showing radiation recall dermatitis after the administration of sorafenib [15,16]. In our case, sorafenib was administered at two months after the completion of PBT because of pulmonary metastasis. Myositis occurred at four months after the start of sorafenib treatment. Previous literatures reported the onset of radiation recall dermatitis after the initiation of sorafenib as 7 to 14 days which is shorter than the present case. The differences in the onsets of the phenomena might result from various factors such as difference in sensitivity according to the tissue, RT regimen, or the interval between the RT and sorafenib treatment. Although there is no report relevant to radiation recall myositis induced by sorafenib or regorafenib specifically, our case implies the probability of radiation recall myositis associated with the targeted agents and further studies elucidating this mechanism would be required.
- While the pain induced by radiation myositis was very severe, it was well controlled by steroid in our case. Previous literatures also reported that the pain by myositis was well controlled by steroid and/or non-steroidal anti-inflammatory drugs (NSAID) [8]. The discontinuation of the causative drugs also can be helpful for radiation recall myositis [11]. Because radiographic change usually precedes the presentation of symptoms, close observation would be required. When radiologic findings representing muscle edema such as increased T2 signal with bandlike demarcation from nonirradiated tissue in magnetic resonance image after high dose RT is shown [17], one should consider the possibility of radiation myositis and early intervention may be warranted.
- In conclusion, dose escalation by PBT can induce myositis adjacent to the treatment area. Sorafenib after PBT may be one of the causes for radiation myositis as a radiation recall phenomenon. Therefore, effort to reduce the dose in muscles adjacent to target volumes and careful observation regarding radiation recall myositis after use of sorafenib are required. Steroid and/or NSAID are found to be effective in the control of radiation myositis symptoms.
DISCUSSION
Figure 1.Initial magnetic resonance images showing huge hepatocellular carcinoma (HCC) with multiple nodular HCCs in the right lobe (arrows). A-P, arterial phase; D-P, delayed phase.
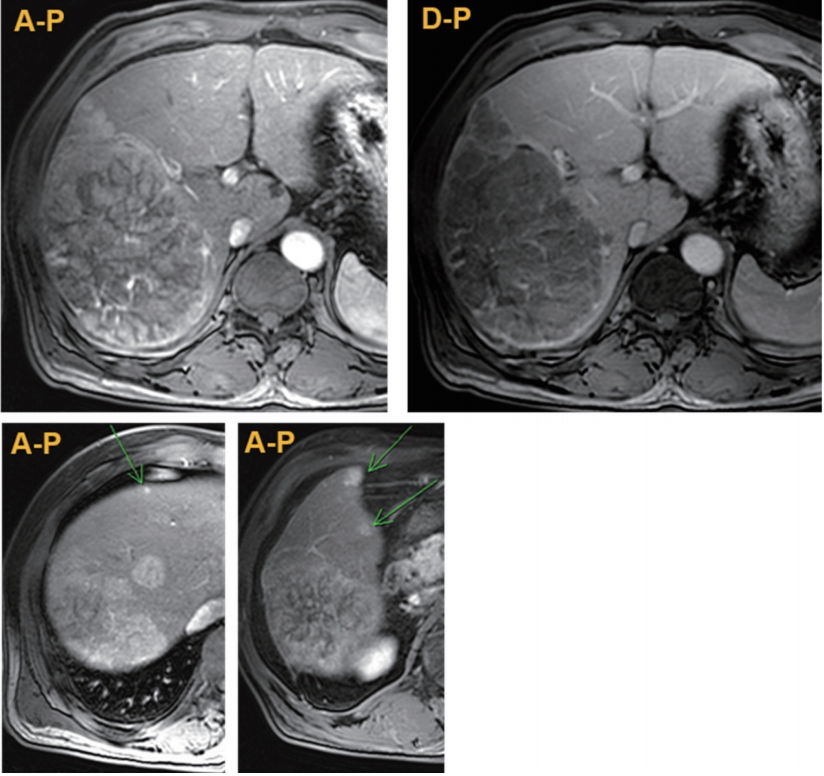

Figure 2.Isodose lines of proton beam therapy. The isodose lines show 30% to 100% of prescribed dose, 72.6 cobalt gray equivalents in 10 fractions.
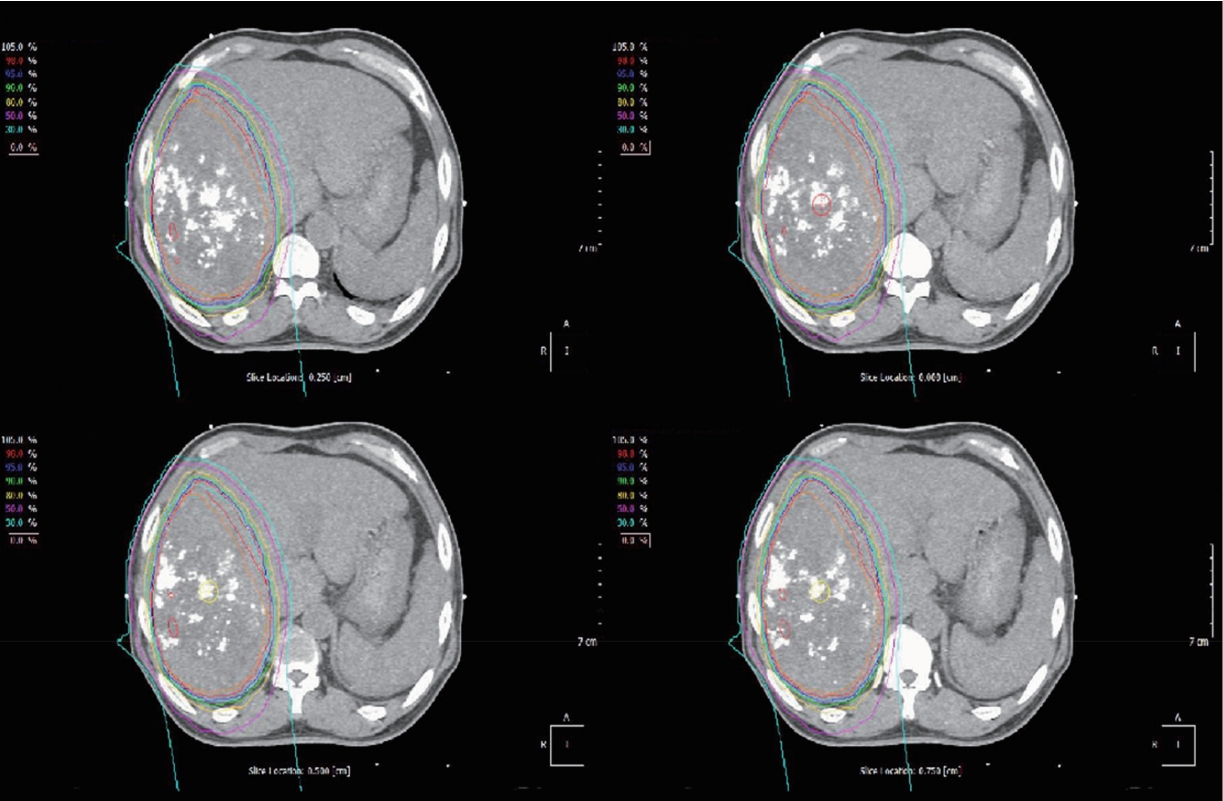

Figure 3.Dose-volume histogram of proton beam therapy. The curve with dark brown color represents the dose volume histogram for chest wall. Maximum dose on chest wall is calculated as 71.1 cobalt gray equivalents (CGE), while dose for target volume reaches 72.6 CGE.


Figure 4.Serial computed tomography (CT) findings focusing on main hepatic mass and the chest wall (from left to right). (A) After the first transarterial chemoembolization (TACE), prior to the initiation of proton beam therapy (PBT). (B) A month after combination of TACE and PBT. The second TACE was performed afterwards. (C) Four months after completion of PBT. (D) CT scan taken 6 months after completion of PBT shows the swollen chest wall with low attenuation (inside the yellow ellipse).
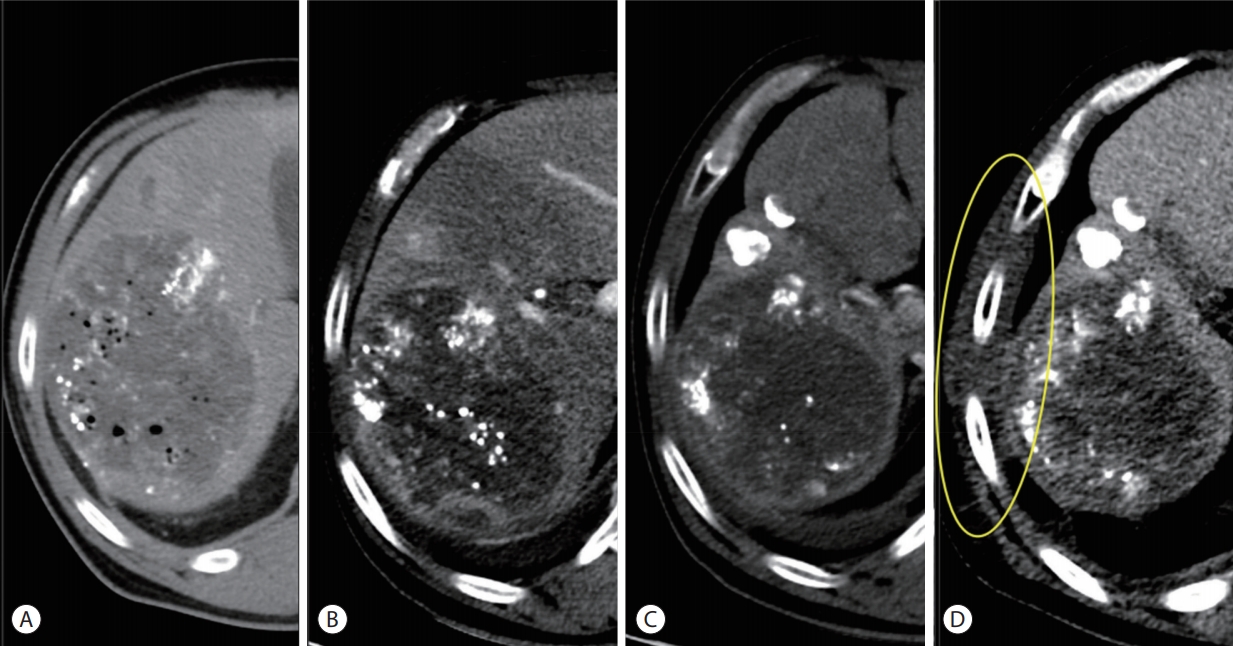

Figure 5.Serial computed tomography (CT) scans showing improvement of radiation myositis (from left to right). (A) Six months after the proton beam therapy (PBT). (B) Eight months after PBT, CT revealed markedly decreased swelling of chest wall. (C) Nine months after PBT.

- 1. Yoo GS, Yu JI, Park HC. Proton therapy for hepatocellular carcinoma: current knowledges and future perspectives. World J Gastroenterol 2018;24:3090−3100.ArticlePubMedPMC
- 2. Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys 2013;87:22−32.ArticlePubMed
- 3. Choi SH, Seong J. Strategic application of radiotherapy for hepatocellular carcinoma. Clin Mol Hepatol 2018;24:114−134.ArticlePubMedPMCPDF
- 4. Kanemoto A, Mizumoto M, Okumura T, Takahashi H, Hashimoto T, Oshiro Y, et al. Dose-volume histogram analysis for risk factors of radiation-induced rib fracture after hypofractionated proton beam therapy for hepatocellular carcinoma. Acta Oncol 2013;52:538−544.ArticlePubMed
- 5. Yeung R, Bowen SR, Chapman TR, MacLennan GT, Apisarnthanarax S. Chest wall toxicity after hypofractionated proton beam therapy for liver malignancies. Pract Radiat Oncol 2018;8:287−293.ArticlePubMed
- 6. Welsh JS, Torre TG, DeWeese TL, O'Reilly S. Radiation myositis. Ann Oncol 1999;10:1105−1108.ArticlePubMedPDF
- 7. Gillette EL, Mahler PA, Powers BE, Gillette SM, Vujaskovic Z. Late radiation injury to muscle and peripheral nerves. Int J Radiat Oncol Biol Phys 1995;31:1309−1318.ArticlePubMed
- 8. Lockney DT, Jia AY, Lis E, Lockney NA, Liu C, Hopkins B, et al. Myositis following spine radiosurgery for metastatic disease: a case series. J Neurosurg Spine 2018;28:416−421.ArticlePubMedPMC
- 9. Borroni G, Vassallo C, Brazzelli V, Martinoli S, Ardigò M, Alessandrino PE, et al. Radiation recall dermatitis, panniculitis, and myositis following cyclophosphamide therapy: histopathologic findings of a patient affected by multiple myeloma. Am J Dermatopathol 2004;26:213−216.ArticlePubMed
- 10. Burstein HJ. Side effects of chemotherapy. Case 1. Radiation recall dermatitis from gemcitabine. J Clin Oncol 2000;18:693−694.ArticlePubMed
- 11. Maeng CH, Park JS, Lee SA, Kim DH, Yun DH, Yoo SD, et al. Radiation recall phenomenon presenting as myositis triggered by carboplatin plus paclitaxel and related literature review. J Cancer Res Ther 2014;10:1093−1097.ArticlePubMed
- 12. Shrimali RK, McPhail NJ, Correa PD, Fraser J, Rizwanullah M. Trastuzumab-induced radiation recall dermatitis--first reported case. Clin Oncol (R Coll Radiol) 2009;21:634−635.ArticlePubMed
- 13. Kang SK. Images in clinical medicine. Radiation recall reaction after antimicrobial therapy. N Engl J Med 2006;354:622. ArticlePubMed
- 14. Phillips TL, Fu KK. Quantification of combined radiation therapy and chemotherapy effects on critical normal tissues. Cancer 1976;37(2 Suppl): 1186−1200.ArticlePubMed
- 15. Kim GE, Song HS, Ahn KJ, Kim YS. Radiation recall dermatitis triggered by sorafenib after radiation therapy for hepatocellular carcinoma. Radiat Oncol J 2017;35:289−294.ArticlePubMedPMCPDF
- 16. Mehta K, Kaubisch A, Tang J, Pirlamarla A, Kalnicki S. Radiation recall dermatitis in patients treated with sorafenib. Case Rep Oncol Med 2018;2018:2171062. ArticlePubMedPMCPDF
- 17. Smitaman E, Flores DV, Mejía Gómez C, Pathria MN. MR imaging of atraumatic muscle disorders. Radiographics 2018;38:500−522.ArticlePubMed
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- Pectoralis Major Radiation Myonecrosis After Lung Stereotactic Body Radiation Therapy
Jason Gurewitz, Anand Mahadevan, Benjamin T. Cooper
Practical Radiation Oncology.2023;[Epub] CrossRef - Current role of proton beam therapy in patients with hepatocellular carcinoma
Gyu Sang Yoo, Jeong Il Yu, Hee Chul Park
International Journal of Gastrointestinal Intervention.2021; 10(4): 175. CrossRef
 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation
- Download Citation
- Close
- Related articles
-
- Complications of immunotherapy in advanced hepatocellular carcinoma
- A multidisciplinary approach with immunotherapies for advanced hepatocellular carcinoma
- The role of lenvatinib in the era of immunotherapy of hepatocellular carcinoma
- Prediction of Post-resection Prognosis Using the ADV Score for Huge Hepatocellular Carcinomas ≥13 cm

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION


 Follow JLC on Twitter
Follow JLC on Twitter