- Page Path
- HOME > Articles and issues
Original Articles
- Superselective Ablative Chemo-ethanol Embolization for Recurrent Single Hepatocellular Carcinoma: A Six-Month Outcome Analysis
- Jae Hwan Lee, Kun Young Kim, Chong-ho Lee, Minuk Kim, Chang Jin Yoon
- Received April 1, 2024 Accepted May 8, 2024 Published online May 14, 2024
- DOI: https://doi.org/10.17998/jlc.2024.05.08 [Accepted]
- 134 Views
- 2 Downloads
-
 Abstract
Abstract
 PDF
PDF - Background/Aim
To evaluate the safety and effectiveness of superselective ablative chemoethanol embolization (SACE) for the treatment of patients with recurrent single hepatocellular carcinoma (rHCC).
Materials and Methods
This retrospective study included 22 patients (19 men, median age 63 [range 38-86 y]) with Child-Pugh class of A/B/C (16/3/3) that underwent SACE between January and June 2023 for recurrent single HCCs measuring ≤ 5 cm in diameter using a mixture of 99% Ethanol and ethiodized oil/doxorubicin emulsion. The primary endpoint was the 6-month tumor response, and the secondary endpoints were the 1-month tumor response and treatment-related safety. This study was approved by our institutional review board, and the requirement for informed consent was waived.
Results
SACE was successfully performed in 22 (95.2%) patients. The complete response rates at 1-month and 6-month after treatment were 100% and 83.3%, respectively. At 6-month, local tumor progression occurred in one patient and intrahepatic distant metastasis was found in 6 (30%) patients. No 6-month mortalities were reported. No adverse events greater than grade 2 or laboratory deteriorations were observed. Biliary complications or liver abscesses were not observed.
Conclusion
SACE for a single rHCC was highly effective in achieving a favorable 6-month tumor response and showed acceptable adverse events. However, further prospective studies are required to verify these findings.

- Re-assessing the diagnostic value of the enhancing “capsule” in hepatocellular carcinoma imaging
- Jae Seok Bae, Jeong Min Lee, Bo Yun Hur, Jeongin Yoo, Sae-Jin Park
- Received March 8, 2024 Accepted May 1, 2024 Published online May 8, 2024
- DOI: https://doi.org/10.17998/jlc.2024.05.01 [Accepted]

- 282 Views
- 7 Downloads
-
 Abstract
Abstract
 PDF
PDF - Background/Aims
The enhancing “capsule” (EC) in hepatocellular carcinoma (HCC) diagnosis has received varying degrees of recognition across major guidelines. This study aimed to assess the diagnostic utility of EC in HCC detection.
Methods
We retrospectively analyzed patients who underwent pre-surgical computed tomography (CT) and hepatobiliary agent-enhanced magnetic resonance imaging (HBA-MRI) between January 2016 and December 2019. A single hepatic tumor was confirmed based on the pathology of each patient. Three radiologists independently reviewed the images according to the Liver Imaging Reporting and Data System (LIRADS) v2018 criteria and reached a consensus. Interobserver agreement for EC before reaching a consensus was quantified using Fleiss κ statistics. The impact of EC on the LI-RADS classification was assessed by comparing the positive predictive values for HCC detection in the presence and absence of EC.
Results
In total, 237 patients (median age, 60 years; 184 men) with 237 observations were included. The interobserver agreement for EC detection was notably low for CT (κ=0.169) and HBA-MRI (κ=0.138). The presence of EC did not significantly alter the positive predictive value for HCC detection in LI-RADS category 5 observations on CT (94.1% [80/85] vs. 94.6% [88/93], P=0.886) or HBA-MRI (95.7% [88/92] vs. 90.6% [77/85], P=0.178).
Conclusions
The diagnostic value of EC in HCC diagnosis remains questionable, given its poor interobserver agreement and negligible impact on positive predictive values for HCC detection. This study challenges the emphasis on EC in certain diagnostic guidelines and suggests the need to re-evaluate its role in HCC imaging.

- Inter-reader Agreement for CT/MRI LI-RADS Category M Imaging Features: A Systematic Review and Meta-analysis
- Dong Hwan Kim, Sang Hyun Choi
- Received March 11, 2024 Accepted April 5, 2024 Published online April 15, 2024
- DOI: https://doi.org/10.17998/jlc.2024.04.05 [Accepted]

- 710 Views
- 19 Downloads
-
 Abstract
Abstract
 PDF
PDF - Backgrounds/Aims
To systematically evaluate inter-reader agreement in the assessment of individual Liver Imaging Reporting and Data System (LI-RADS) category M (LR-M) imaging features in computed tomography/magnetic resonance imaging (CT/MRI) LI-RADS v2018, and to explore the causes of poor agreement in LR-M assignment.
Methods
Original studies reporting inter-reader agreement for LR-M features on multiphasic CT or MRI were identified using the MEDLINE, EMBASE, and Cochrane databases. The pooled kappa coefficient (κ) was calculated using the DerSimonian–Laird random-effects model. Heterogeneity was assessed using Cochran’s Q test and I2 statistics. Subgroup meta-regression analyses were conducted to explore the study heterogeneity.
Results
In total, 24 eligible studies with 5,163 hepatic observations were included. The pooled κ values were 0.72 (95% confidence interval, 0.65–0.78) for rim arterial phase hyperenhancement, 0.52 (0.39–0.65) for peripheral washout, 0.60 (0.50–0.70) for delayed central enhancement, 0.68 (0.57–0.78) for targetoid restriction, 0.74 (0.65–0.83) for targetoid transitional phase/hepatobiliary phase appearance, 0.64 (0.49–0.78) for infiltrative appearance, 0.49 (0.30–0.68) for marked diffusion restriction, and 0.61 (0.48–0.73) for necrosis or severe ischemia. Substantial study heterogeneity was observed for all LR-M features (Cochran's Q test: p < 0.01; I2 ≥ 89.2%). Studies with a mean observation size of <3 cm, those performed using 1.5-T MRI, and those with multiple image readers, were significantly associated with poor agreement of LR-M features.
Conclusions
The agreement for peripheral washout and marked diffusion restriction was limited. The LI-RADS should focus on improving the agreement of LR-M features.

- Outcomes of Liver Resection and Transarterial Chemoembolization in Patients with Multinodular BCLC-A Hepatocellular Carcinoma
- Jiwon Yang, Won-Mook Choi, Danbi Lee, Ju Hyun Shim, Kang Mo Kim, Young-Suk Lim, Han Chu Lee, Deok-Bog Moon, Dong-Hwan Jung, Jonggi Choi
- Received March 3, 2024 Accepted March 25, 2024 Published online April 3, 2024
- DOI: https://doi.org/10.17998/jlc.2024.03.25 [Accepted]
- 542 Views
- 42 Downloads
-
 Abstract
Abstract
 PDF
PDF - Background
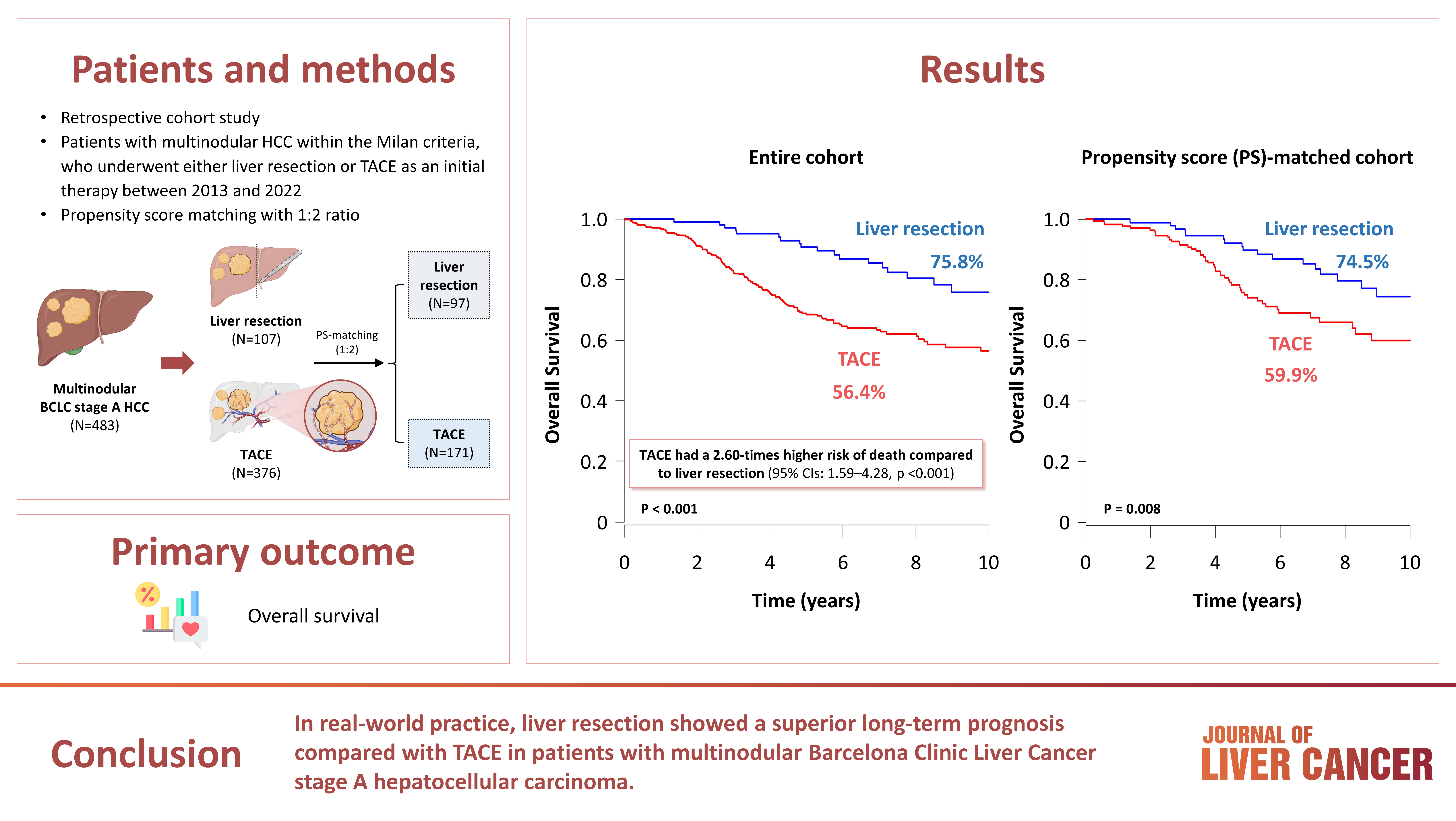
This study aimed to compare the outcomes of liver resection (LR) and transarterial chemoembolization (TACE) in patients with multinodular hepatocellular carcinoma (HCC) within the Milan criteria who were not eligible for liver transplantation.
Methods
We retrospectively analyzed 483 patients with multinodular HCC within the Milan criteria, who underwent either LR or TACE as an initial therapy between 2013 and 2022. The overall survival (OS) in the entire population and recurrence-free survival (RFS) in patients who underwent LR and TACE and achieved a complete response were analyzed. Propensity score (PS) matching analysis was also used for a fair comparison of outcomes between the two groups.
Results
Among the 483 patients, 107 (22.2%) and 376 (77.8%) underwent LR and TACE, respectively. The median size of the largest tumor was 2.0 cm, and 72.3% of the patients had two HCC lesions. The median OS and RFS were significantly longer in the LR group than in the TACE group (p <0.01 for both). In the multivariate analysis, TACE (adjusted hazard ratio [aHR], 1.81 and aHR, 2.41) and large tumor size (aHR, 1.43 and aHR, 1.44) were significantly associated with worse OS and RFS, respectively. The PS-matched analysis also demonstrated that the LR group had significantly longer OS and RFS than the TACE group (PS <0.05).
Conclusion
In this study, LR showed better OS and RFS than TACE in patients with multinodular Barcelona Clinic Liver Cancer stage A HCC. Therefore, LR can be considered an effective treatment option for these patients.


 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION
 First
First Prev
Prev



 Follow JLC on Twitter
Follow JLC on Twitter