A case of nearly complete response in hepatocellular carcinoma with disseminated lung metastasis by combination therapy of nivolumab and ipilimumab after treatment failure of atezolizumab plus bevacizumab
Article information
Abstract
Recently, the efficacy of immuno-oncologic agents for advanced hepatocellular carcinoma (HCC) has been proven in several trials. In particular, atezolizumab with bevacizumab (AteBeva), as a first-line therapy for advanced HCC, has shown tremendous advances in the IMBrave150 study. However, second or third-line therapy after treatment failure with AteBeva has not been firmly established. Moreover, clinicians have continued their attempts at multidisciplinary treatment that includes other systemic therapy and radiotherapy (RT). Here, we report a case that showed a near complete response (CR) of lung metastasis to nivolumab with ipilimumab therapy after achieving a near CR of intrahepatic tumor using sorafenib and RT in a patient with advanced HCC who had experienced treatment failure of AteBeva.
INTRODUCTION
The most frequent primary malignant tumor of the liver is hepatocellular carcinoma (HCC), which also accounts for the second-most cancer-related deaths worldwide.1 Surgical treatment and local ablation are the only treatment methods leading to a complete cure. However, treatment options are limited for patients with advanced HCC because of poor liver function and extensive metastasis, so it is often difficult to achieve a cure.2
Recently, numerous clinical trials on systemic therapy have been conducted to achieve the best results for advanced HCC; in particular, studies on immuno-oncologic agents are actively ongoing. Recently, the IMBrave150 trial demonstrated that patients receiving a combination therapy of atezolizumab plus bevacizumab (AteBeva) had better overall survival (OS) and median progression-free survival (PFS) than people treated with sorafenib.3
However, second or third-line therapy after treatment failure with AteBeva has not been firmly established and requires further clinical trials. Therefore, clinicians have continued their attempts at multidisciplinary treatment that includes other systemic therapy and radiotherapy (RT). Here, we report a case that showed a near complete response (CR) of lung metastasis to nivolumab with ipilimumab (Nivo+Ipi) therapy after achieving a near CR of intrahepatic tumor using sorafenib and RT in a patient with advanced HCC who had experienced treatment failure with AteBeva.
CASE REPORT
A 44-year-old man with a large liver tumor was admitted to our hospital from a nearby clinic. Magnetic resonance imaging (MRI) revealed approximately 21.9 cm of infiltrative HCC with right portal vein tumor thrombosis (PVTT) and multiple lung metastases (Figs. 1, 2A). The initial blood tests showed a white blood cell count of 8,080/mm3, hemoglobin level of 16.6 g/dL, platelet count of 200,000/µL, aspartate aminotransferase concentration of 266 IU/L, alanine aminotransferase concentration of 17 IU/L, total protein level of 6.6 g/dL, total albumin level of 3.6 g/dL, prothrombin time of 84%, total bilirubin concentration of 1.9 mg/dL, and hepatitis B virus DNA 2,600 IU/mL with hepatitis B e-antigen positivity. Entecavir 0.5 mg was administered to the patient every day. The initial alpha-fetoprotein (AFP) level was above 100,000 ng/mL, and the protein level induced by the absence of vitamin K or antagonist-II (PIVKA-II) was 23,230 mAU/mL (Fig. 3). Liver function was preserved with a Child-Turcotte-Pugh score of 5. The Eastern Cooperative Oncology Group (ECOG) performance status score was 1. Based on typical MRI features, the patient was identified as having advanced-stage HCC Barcelona Clinic Liver Cancer (BCLC) C advanced stage (modified Union for International Cancer Control T4N1M1, stage IV B).
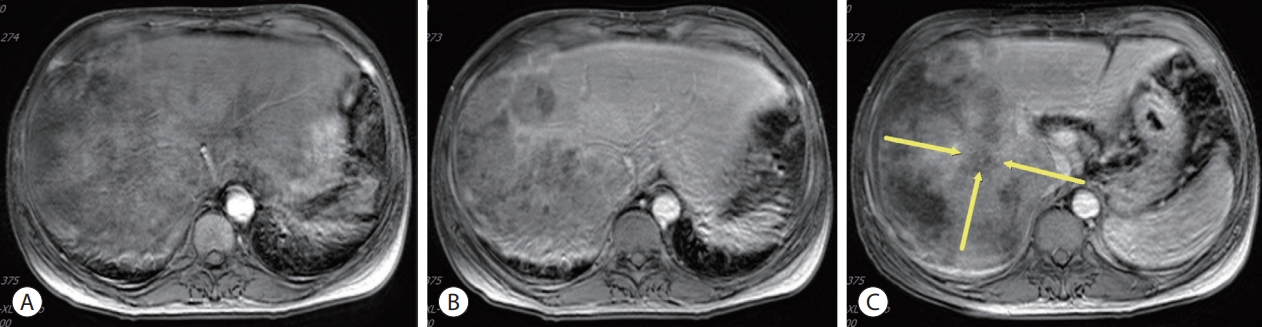
Initial liver magnetic resonance imaging findings. (A) Approximately 21.9 cm of the heterogeneously enhanced mass involving the right lobe of the liver with multifocal arterial enhancement. (B) Delayed washout. (C) Obliteration of the right portal vein (yellow arrows).
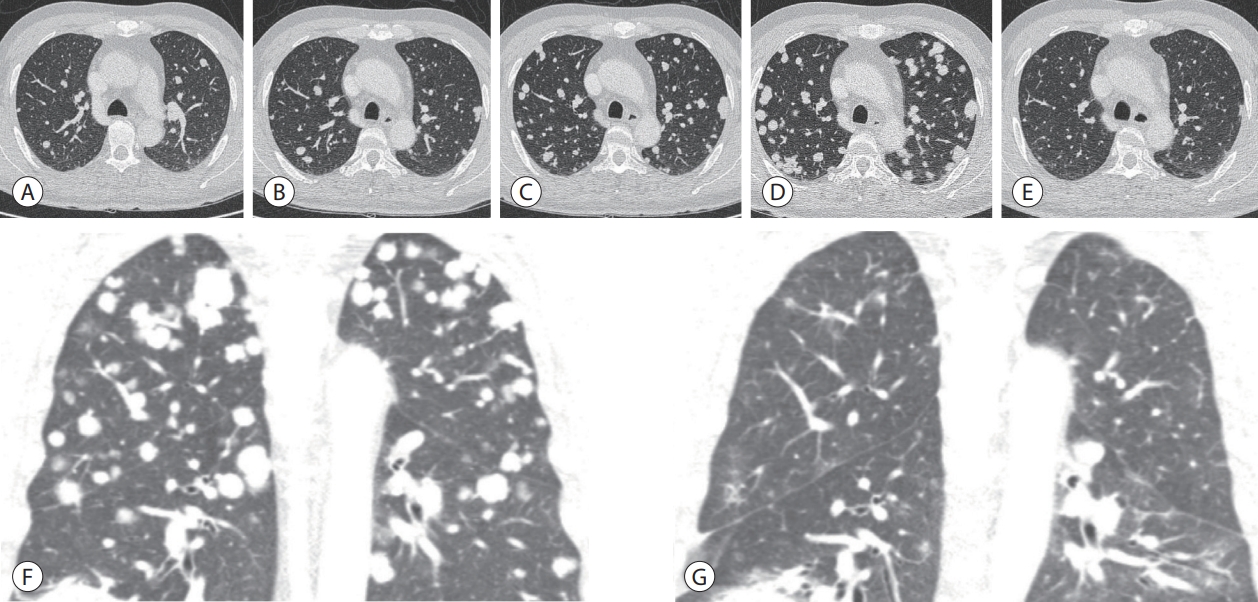
Chest contrast-enhanced computed tomography. (A) Before AteBeva therapy. (B) After 3 months of AteBeva therapy. (C) After 2 months of sorafenib plus radiation therapy. (D, E) After 3 months of regorafenib therapy. (F, G) After 3 months of nivolumab plus ipilimumab therapy. AteBeva, atezolizumab plus bevacizumab.
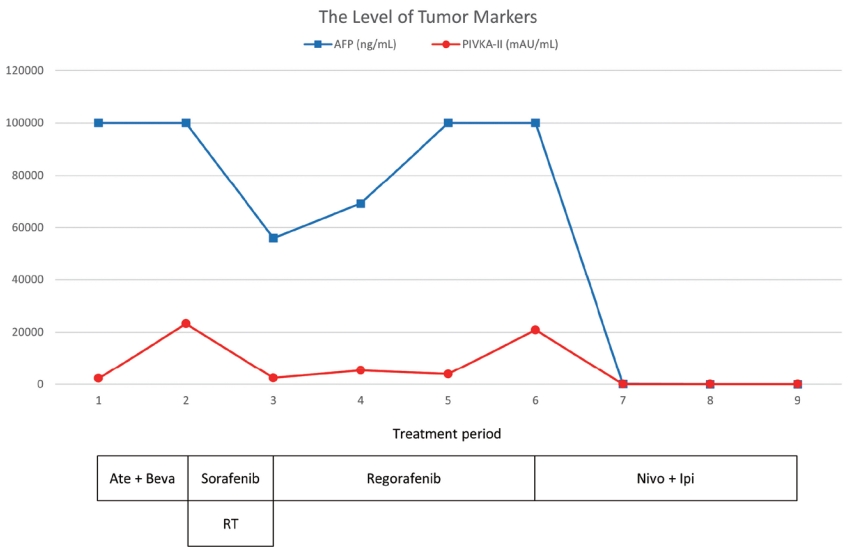
The clinical course of serum AFP and PIVKA-II levels and the timing of the therapeutic methods. AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence-II; Ate+Beva, atezolizumab plus bevacizumab; RT, radiation therapy; Nivo+Ipi, nivolumab plus ipilimumab.
The patient was treated with AteBeva according to several guidelines. Atezolizumab (Ticentriq® 1,200 mg per dose, Roche International LLC., Basel, Switzerland) and bevacizumab (Avastin® 15 mg/kg per dose, Genentech, South San Francisco, CA, USA) were administered every 3 weeks on the same day. Following the fourth cycle of AteBeva, liver MRI revealed an enormous heterogeneous mass involving the right lobe of the liver that was slightly larger (approximately 22.1 cm) than that on the prior liver scan (Fig. 4A). Furthermore, chest computed tomography (CT) revealed that both lung metastases had grown in size and developed new lesions compared to the previous scan (Fig. 2B). After AteBeva failure as the first systemic therapy, the patient was started on 400 mg sorafenib orally, twice daily. We simultaneously performed RT (25 Gy/12 fx) for PVTT over 12 days (Fig. 5).
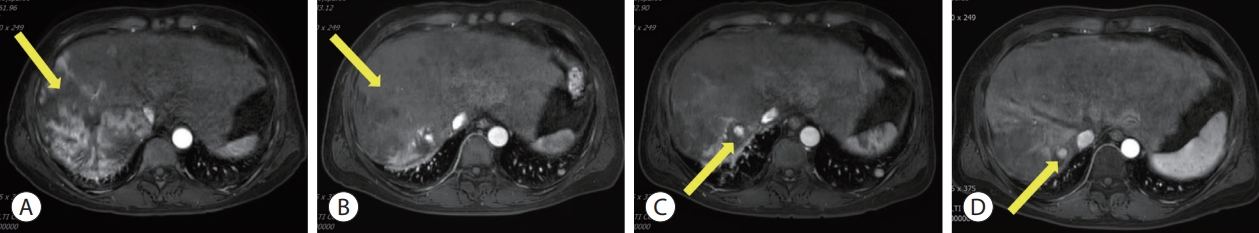
Follow-up liver magnetic resonance imaging. (A) After 3 months of AteBeva therapy, slightly increased size (about 22.1 cm) and number of other HCCs in the right lobe of the liver (yellow arrow). (B) After 2 months of sorafenib plus radiation therapy, reduced size by more than 30% of the enormous heterogeneous mass including the right lobe of the liver (yellow arrow). (C) After 3 months of regorafenib therapy, arterial enhancement nearly disappeared and only a single nodular arterial enhancing lesion was slightly increased (yellow arrow). (D) After 3 months of nivolumab plus ipilimumab therapy, the size of the single nodular arterial enhancing lesion was slightly decreased (yellow arrow). AteBeva, atezolizumab plus bevacizumab; HCC, hepatocellular carcinoma.
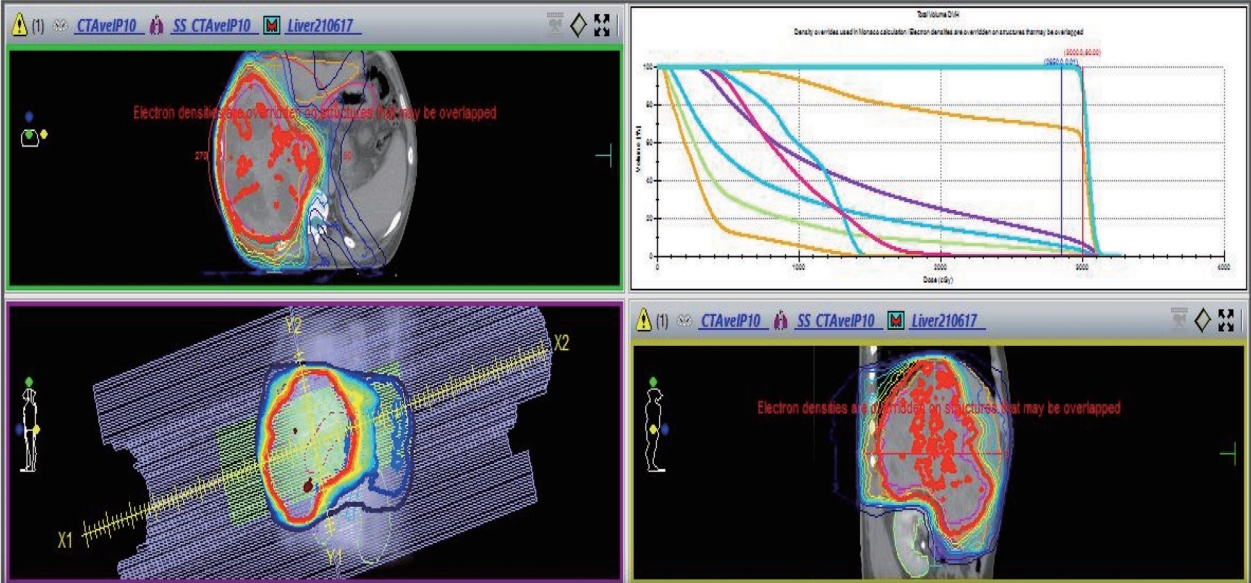
Simultaneous integrated boost radiotherapy and intensity-modulated radiation treatment. Planning target volume 25 Gy with 12 fractions is the required dose for the specified target volumes.
Follow-up MRI that was conducted 2 months after commencing sorafenib plus RT showed a partial response, with decreasing levels of AFP and PIVKA-II (Figs. 3, 4B). However, the number of lung metastases increased on the chest CT scan (Fig. 2C). The patient started taking 160 mg regorafenib orally once a day for 3 weeks on and 1 week off.
Three months after starting regorafenib, a follow-up MRI showed that the arterial enhancement of the large heterogeneous mass involving the right lobe of the liver had nearly disappeared, and only one nodular arterial enhancing lesion had slightly increased (Fig. 4C). However, the lung metastases markedly progressed with increased AFP and PIVKA-II on the chest CT scan (Figs. 2D, E, 3). We then decided to treat the patient with nivolumab 3 mg/kg plus ipilimumab 1 mg/kg, administered every 3 weeks (four doses), followed by nivolumab 240 mg every 2 weeks as the fourth-line systemic therapy. Six months after initiating Nivo+Ipi therapy, the AFP and PIVKA-II levels normalized (Fig. 3), the single viable portion of intrahepatic HCC slightly decreased, and the multiple lung metastases nearly disappeared (Figs. 2F, G, 4D). We continued treating the patient with nivolumab monotherapy every 2 weeks. The patient underwent 26 cycles of nivolumab therapy for 56 weeks and showed no signs of disease progression.
DISCUSSION
Most HCC is advanced at the time of diagnosis and frequently incurable. Consequently, therapeutic options are restricted to palliative care.4 Sorafenib has long been used as a palliative treatment for patients with advanced HCC based on beneficial results in the SHARP trial.5 Combination therapy with AteBeva was established as the initial treatment after demonstrating outstanding outcomes in the IMBrave150 study.
AteBeva had a 12-month OS rate of 67.2% (95% confidence interval [CI], 61.3–73.1), whereas sorafenib had an OS rate of 54.6% (95% CI, 45.2–64.0). In the respective groups, the median PFS was 6.8 months (95% CI, 5.7–8.3) and 4.3 months (95% CI, 4.0–5.6) (hazard ratio for disease progression or death, 0.59; 95% CI, 0.47–0.76; P=0.001).3
However, second or third-line therapy after treatment failure with AteBeva has not been firmly established. Although the updated BCLC guidelines recommend carbozantinib as a salvage therapy after AteBeva treatment failure, the recommendation has not been clearly established by a well-designed clinical trial.6
Nivo+Ipi treatment has recently been demonstrated as effective for advanced HCC second-line treatment. As part of CheckMate040, a clinical trial to determine the effect of secondline treatment with Nivo+Ipi in patients with HCC who experienced failed sorafenib treatment, a meaningful response rate (RR) and durable response were shown. The investigator-assessed RR was 32% in arm A (four doses of nivolumab 1 mg/kg + ipilimumab 3 mg/kg every 3 weeks followed by nivolumab 240 mg every 2 weeks), although the median duration of response was not met.7 Based on these results, the 2022 Korean guidelines proposed that immune checkpoint inhibitors with different targets, such as Nivo+Ipi, may be considered as salvage therapies for patients who experienced treatment failure with immunologic agents.4 Indeed, in some retrospective studies, authors have reported a median OS of Nivo+Ipi treatment between 7.4 and 10.9 months and a RR between 16% and 30%, suggesting that Nivo+Ipi treatment may be effective in advanced HCC after failure of the combination therapy AteBeva.8,9
RT has been used as palliative treatment for HCC in several clinical settings. RT is usually performed alone; however, it is sometimes performed concurrently with systemic therapy, with a synergistic effect expected from such a combination. Recent clinical studies have shown that patients with advanced HCC who received sorafenib plus RT had a greater 1-year survival rate than those who received sorafenib alone.10,11 In this case, we suggest that there might be a synergistic effect of Nivo+Ipi treatment with RT. However, we think the possibility is low that the suggestion is true because there was a time difference of nearly 6 months between the Nivo+Ipi therapy and RT. Therefore, further research on the combined treatment of Nivo+Ipi plus RT is needed.
In conclusion, we report a case that showed a near CR of lung metastases using Nivo+Ipi therapy after achieving a near CR of intrahepatic tumor using sorafenib and RT in a patient with advanced HCC patient who had experienced treatment failure with AteBeva. We need to observe further real-life data in Korea regarding salvage therapy after treatment failure with AteBeva.
Notes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Ethics Statement
The present case report was approved by the Institutional Review Board (IRB) of the Dongnam Institute of Radiological & Medical Sciences, Busan, Korea (IRB number: D-2108-019-002).
Funding Statement
None.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed for this case report.
Author Contribution
Conceptualization: HJK, JWI, KJJ, WJ
Visualization: HJK
Writing–original draft: HJK
Writing–review & editing: SYH, JWI
