The effects of immune checkpoint modulators on the clinical course of patients with resectable hepatocellular carcinoma
Article information
Abstract
Background/Aim
Immune checkpoint proteins regulating T-cell mediated anti-tumor immunity have been reported to affect clinical outcomes in multiple malignancies. This study aimed to investigate the prognostic effect of histological expression of immune checkpoint proteins in patients with resected hepatocellular carcinoma (HCC).
Methods
A total of 221 patients with HCC who underwent curative resection were included. Expression of programmed-cell death ligand-1 (PD-L1) in tumor cells (tPD-L1) and tumor infiltrating mononuclear cells (TIMCs) (iPD-L1), programmed-cell death-1 in TIMCs (iPD-1), and cytotoxic T lymphocyte antigen-4 in TIMCs (iCTLA-4) were measured immunohistochemically.
Results
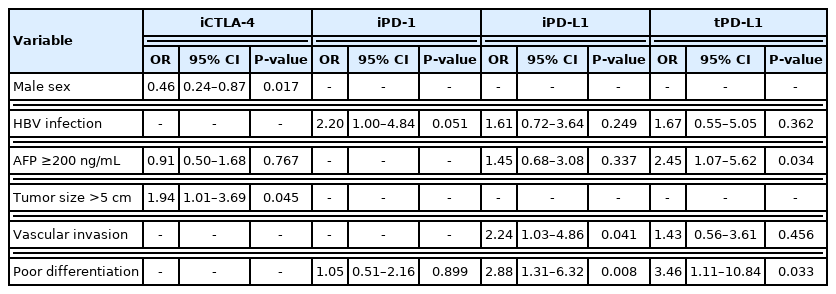
Histo-positivity for iCTLA-4, iPD-1, iPD-L1, and tPD-L1 was 32.1%, 42.5%, 35.3%, and 14.9%, respectively. Multivariate logistic analyses revealed that male sex and tumor >5 cm were variables related to iCTLA-4 positivity (odds ratio [OR], 0.46 and 1.94, respectively; P<0.05). Poor differentiation was related to PD-L1 expression in both tumor cells and TIMCs (OR, 2.88 and 3.46, respectively; P<0.05). Microvascular invasion was significantly associated only with iPD-L1 (OR, 2.24; P<0.05). In time-dependent outcome analyses, expression of immune checkpoint proteins in TIMCs (i.e., iCTLA-4, iPD-1, and iPD-L1) was significantly related to longer overall survival and non-cancer-related survival (all P<0.05), but not to time-to-recurrence or cancer-specific deaths. Concurrent activation of the PD-1:PD-L1 and CTLA-4 pathways predicted improved outcomes in terms of overall survival and non-cancer related survival (P=0.06 and P=0.03, respectively).
Conclusions
Immune checkpoint proteins upregulated in TIMCs in HCC tissues have individual and additive effects in prolonging the survival of patients, specifically in terms of survival not related to cancer recurrence.
INTRODUCTION
The immune system plays a dual role in cancer.1,2 First, it suppresses tumor growth by destroying cancer cells or inhibiting their growth. Second, it promotes tumor progression by selecting tumor cells that are more likely to survive in an immunocompetent host or by establishing conditions within the tumor microenvironment that may facilitate tumor growth.2,3 Effective antitumor immunity depends on interactions between various T-cell regulatory receptors and ligands including the cytotoxic T lymphocyte antigen-4 (CTLA-4)/B7 and programmed-cell death-1 (PD-1)/programmed-cell death ligand-1 (PD-L1) signaling pathways.2,4
These immune checkpoints are known to regulate different stages and signaling processes of the immune response.3,4 At the initial stage of “priming” of naïve T-cell activation after antigen encounter, CTLA-4:B7 binding blocks stimulatory signals, and stops the development of potentially autoreactive T cells.5,6 In contrast, a major role of the pathway involving PD-1 and its ligand, PD-L1, is to regulate previously activated T cells at the later “effector” stage of immune response.7,8 In the tumor microenvironment, antigen-specific T cells induce PD-1 expression on reactive T lymphocytes and upregulate PD-L1 in tumor cells.8 The subsequent PD-1:PD-L1 interaction results in T-cell exhaustion and immune evasion by tumor cells.3,7 On the other hand, this interaction can limit collateral tissue damage, as observed for example in response to chronic infection by microorganisms such as hepatitis virus. 3,9–12
Previous studies have shown that the immune checkpoint proteins CTLA4, PD-1, and PD-L1 can be used as reliable biomarkers for predicting the clinical behavior of many types of tumor.13–19 These immune molecules are highly expressed in hepatocellular carcinomas (HCCs) that are recognized histologically as immunogenic tumors.20–22 Generally, it has been noted that the chronically inflamed background and dysregulated immune microenvironment in the liver result in the upregulation of checkpoint proteins in HCCs.23 This may make HCC tumors to be plausible candidates for immune checkpoint inhibitor treatment. In addition, hepatitis B and hepatitis C virus infections, two major causes of HCC, have been shown to interfere with antiviral immunity via the immune checkpoint pathways.9,10,12,24 The relationships between the biology of HCC and immunomodulatory proteins is unclear and inconsistent across publications.20–22,25
Since immunotherapy for HCC has become to be one of standard systemic options, we aimed to identify different individual or interactive roles of immune checkpoint proteins as oncomarkers during the long clinical course after surgical resection. We therefore examined the clinical and pathological factors associated with histological expression of immune checkpoint proteins in a series of patients with operable HCC.
METHODS
1. Study patients
This clincopathologic study was based on tissue microarrays of paraffin-embedded samples from a cohort of 221 patients undergoing hepatic resection for HCC with curative intent in our center between 2004 and 2011. All included patients with histology-proven HCC had Child-Pugh A or B liver function without extrahepatic metastasis, gross vascular invasion or concomitant cancers. None received neoadjuvant or adjuvant treatment in the perioperative period. The study protocol was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2011-0931).
Of the 221 patients, 165 (74.7%) were male, and the median age was 56 years. The most common cause of chronic liver disease was hepatitis B virus (HBV) infection (n=160, 72.4%). The majority of patients had solitary tumors (n=198, 89.6%), which ranged from 0.6 to 18.0 cm in maximum diameter. Microvascular invasion and poor differentiation on resected specimens were observed in 26.7% (n=59) and 64.7% (n=143) of the patients, respectively. Disease stage was classified as 0 (n=6, 2.7%), A (n=199, 90.1%), or B (n=16, 7.2%) according to the Barcelona Clinic Liver Cancer staging system. High preoperative alpha-fetoprotein (AFP) levels (≥200 ng/mL) were detected in 36.7% (n=81) of the patients. The clinical and biological features of the series are summarized in Table 1. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) reporting guidelines were followed (Supplementary Table 1).
2. Immunohistochemical staining and evaluation
Serial 4-μm thick sections of formalin-fixed paraffin-embedded samples of HCCs and adjacent tissue were used for immunohistochemical staining. All the slides were processed on an automated immunostaining device (Ventana Medical System, Tucson, AZ, USA), with an OptiView DAB IHC Detection Kit (Ventana Medical System) according to the manufacturer’s instructions. The following primary antibodies were used: anti-PD-L1 (rabbit monoclonal E1L3N, 1/100; Cell Signaling, Danvers, MA, USA), anti-PD-1 (mouse monoclonal ab52587, 1/100; Abcam, Cambridge, UK), and anti-CTLA-4 (mouse monoclonal ab134090, 1/500; Abcam). All immunostaining was independently reviewed by two pathologists (EY, HJK) specialized in liver diseases, who was blinded to clinical outcomes.
PD-L1 expression was assessed in both tumor cells (tPD-L1) and intratumoral inflammatory cells, identified as T cells, macrophages and dendritic cells that infiltrated tumor cell nests (PD-L1 in tumor infiltrating mononuclear cells, iPD-L1). The percentages of cells with surface PD-L1 were scored, and cases with ≥1% tumor cell expression were considered positive.13,15,26 PD-1 expression was only observed in intratumoral lymphocytes, and ≥1% positive cells were regarded as positive.21,27 Samples with any expression of CTLA-4 in tumor infiltrating mononuclear cells (TIMCs) and tumor cells were classified as positive.28,29 Representative images of positive staining of each molecule were shown in Fig. 1.

Presence of immune checkpoint proteins in formalin-fixed paraffin-embedded samples. Positive staining of each molecule is shown as follows (×400): (A) cytotoxic T lymphocyte antigen-4 in tumor-infiltrating mononuclear cells (TIMCs), (B) programmed-cell death-1 in TIMCs, (C) programmed-cell death ligand-1 (PD-L1) in TIMCs, and (D) PD-L1 in tumor cells.
3. Statistical analysis
Continuous variables and proportions were compared using the Mann-Whitney, chi-square, and Fisher’s exact tests, as appropriate. Probabilities of overall survival, cancer-specific survival, non-cancer related survival, and time-to-tumor recurrence were estimated using the Kaplan-Meier method and compared using Cox proportional hazards regression models according to the expression of individual and combinations of immune checkpoints. Death due to HCC progression was considered cancer-specific survival. Hazard ratios were adjusted for age, gender, etiology of liver disease, presence of liver cirrhosis, Child-Pugh class, serum AFP, tumor stage at diagnosis, tumor number, tumor size, presence of vascular invasion, and histologic differentiation. A backward elimination approach involving candidate variables with P-values ≤0.10 in the univariate analysis was used in the multivariable analysis. The associations between clinical and pathological variables and immune checkpoint expression were analyzed by the logistic regression method. Correlations between pairs of immune checkpoints were evaluated by Pearson’s coefficient method. Two-tailed values of P <0.05 were considered statistically significant. Statistical analyses were performed with SPSS 22.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Expression profiles and inter-relationships of immune checkpoint proteins
The immunohistochemical features of the specimens are summarized in Table 1. PD-1 in TIMCs (iPD-1), iPD-L1, and cytotoxic T lymphocyte antigen-4 in TIMCs (iCTLA-4) were expressed in 42.5% (n=94), 35.3% (n=78), and 32.1% (n=71) of the TIMCs, respectively, in the entire 221 samples; and tPD-L1 was positive in 14.9% (n=33) of the tumor cells. No CTLA-4-positive neoplastic cells were detected in any sample.
Univariate and subsequent multivariate logistic regressions showed that poorly differentiated histology was the only variable independently associated with the expression of both iPD-L1 and tPD-L1 (adjusted odds ratio [OR], 2.88; 95% confidence interval [CI], 1.31–6.32; P =0.008 and adjusted OR, 3.46; 95% CI, 1.11–10.84; P =0.033, respectively; Table 2). Microvascular invasion was only significantly related to iPD-L1 expression (adjusted OR, 2.24; 95% CI, 1.03–4.86; P =0.041). iCTLA-4 was associated with male sex (adjusted OR, 0.46; 95% CI, 0.24–0.87; P =0.017), and iPD-1 positivity was not significantly related to clinical or other pathological parameters (Table 2).
In terms of the relationships between different immune checkpoints, Pearson’s correlation analyses revealed that the expression of all three molecules in TIMCs and tumor cells was significantly correlated, with coefficients ranging from 0.282 to 0.437 (all P <0.001, Supplementary Table 2).
2. Survival and recurrence analyses as a function of expression of immune checkpoint proteins
During a median 7.09 years of follow-up (interquartile range [IQR], 5.52–8.93 years), 71 patients (32.1%) died, and 49.3% of the deaths (n=35) were related to HCC progression. In Kaplan-Meier models, 5-year overall survival rates in patients with and without iCTLA-4, iPD-1, iPD-L1, and tPD-L1 expression were 85.9% and 75.3% (P =0.076); 85.1% and 74.1% (P =0.010); 84.6% and 75.5% (P =0.022); and 75.8% and 79.3% (P =0.511) (Fig. 2). After adjustment of confounding covariates with P-values of <0.10 in the univariate analysis, the individual prognostic values of iCTLA-4, iPD-1, and iPD-L1 positivity for overall survival remained significant (adjusted hazard ratio [HR], 0.49; 95% CI, 0.28–0.87; P =0.014; adjusted HR, 0.53; 95% CI, 0.32–0.88; P =0.015; and adjusted HR, 0.52; 95% CI, 0.29–0.91; P =0.023; respectively, Table 3). The adjusted confounding covariates were age, sex, HBV infection, liver cirrhosis, Child-Pugh class, serum AFP, size and number of tumors, microvascular invasion, and poor differentiation. In subsequent survival analyses by specific cause of death, histologic upregulation of any of the three proteins did not influence cancer-related survival (all P >0.05; Table 3, Supplementary Fig. 1). However, positive expression of iCTLA-4, iPD-1, or iPD-L1 was independently associated with reduced mortality from causes other than HCC (HR, 0.39; 95% CI, 0.17–0.90; P =0.028; HR, 0.35; 95% CI, 0.15–0.83; P =0.016; and HR, 0.35; 95% CI, 0.14–0.87; P =0.024, respectively; Table 3, Supplementary Fig. 2).
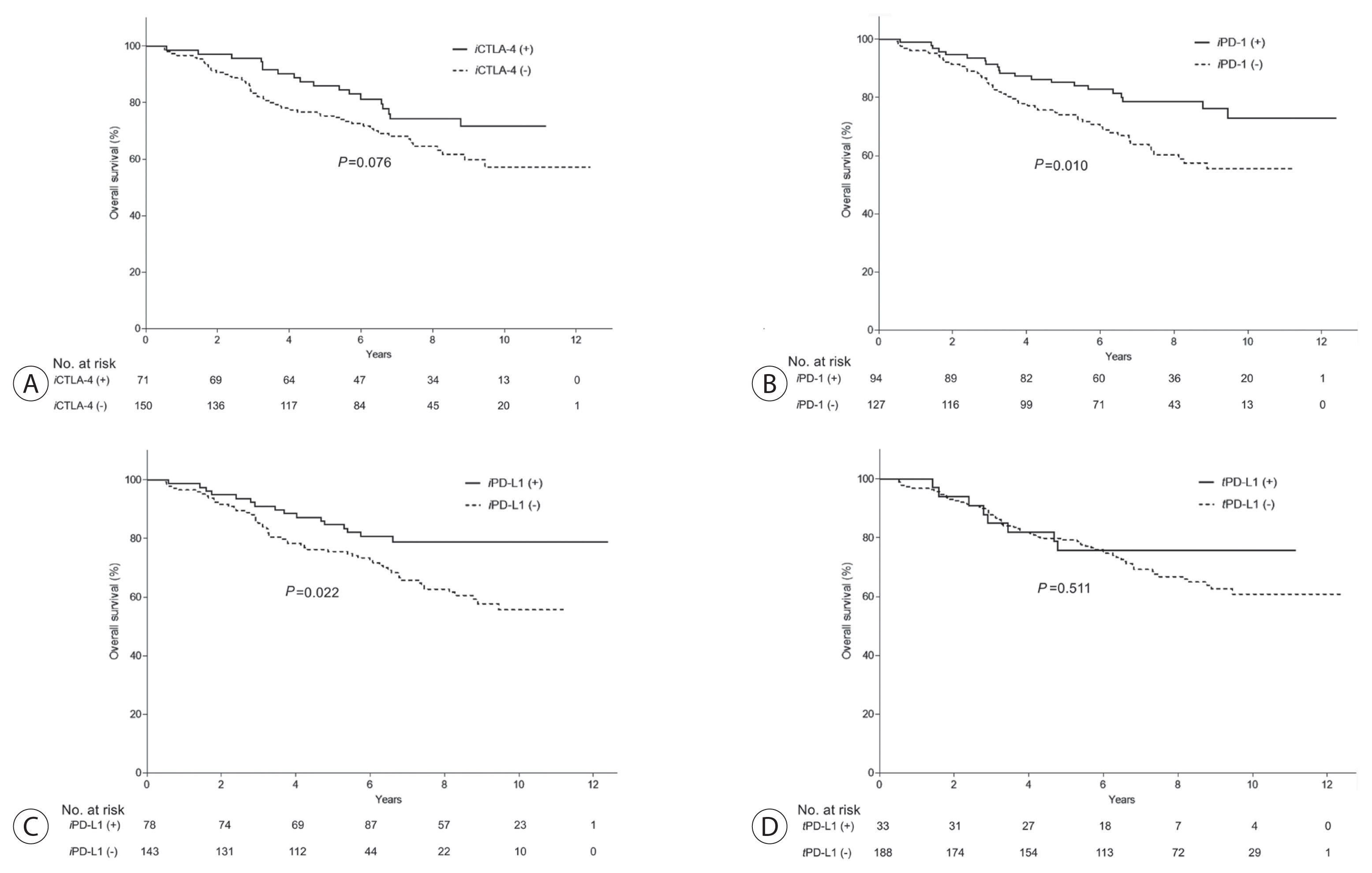
Associations between presence of immune checkpoint proteins and overall survival. Enhanced expression of immune checkpoint molecules in tumor-infiltrating mononuclear cells (A: iCTLA-4, B: iPD-1, and C: iPD-L1) was significantly associated with longer survival of hepatocellular carcinoma patients, whereas tumoral PD-L1 (D: tPD-L1) had no prognostic significance. iCTLA-4, cytotoxic T lymphocyte antigen-4 in tumor-infiltrating mononuclear cells; iPD-1, programmed-cell death-1 in tumor-infiltrating mononuclear cells; iPD-L1, programmed-cell death ligand-1 in tumor-infiltrating mononuclear cells; tPD-L1, programmed-cell death ligand-1 in tumor cells.
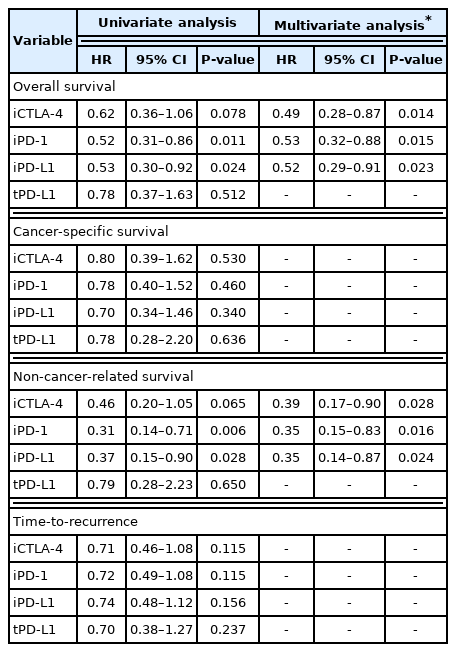
Associations between expression of immune checkpoint proteins and time-dependent outcomes in 221 patients with hepatocellular carcinoma
After resection, HCC reoccurred in 45.7% of the total patients (n=101). Median time-to-recurrence was 4.6 years (IQR, 1.1–6.7 years). Microscopic vessel invasion and multiple tumor number were significantly associated with shorter time-to-recurrence after resection (adjusted HR, 0.53; 95% CI, 0.32–0.88 and adjusted HR, 0.52; 95% CI, 0.29–0.91, respectively; both P <0.05). There were no correlations between expression of immune checkpoints and time-to-recurrence (all P >0.05; Table 3, Fig. 3).

Expression of immune checkpoint proteins and time-to-recurrence. None of the immune checkpoint proteins was significantly associated with time-to-recurrence in the HCC patients (all P >0.05). (A) iCTLA4, (B) iPD-1, (C) iPD-L1, and (D) tPD-L1. HCC, hepatocellular carcinoma; iCTLA-4, cytotoxic T lymphocyte antigen-4 in tumor-infiltrating mononuclear cells; iPD-1, programmed-cell death-1 in tumor-infiltrating mononuclear cells; iPD-L1, programmed-cell death ligand-1 in tumor-infiltrating mononuclear cells; tPD-L1, programmed-cell death ligand-1 in tumor cells.
3. Prognostic effect of combined expression of the PD-1/PD-L1 and CTLA-4 pathways
We further examined whether the PD-1/PD-L1 and CTLA-4 axes had a combined effect on survival. The patients were divided into three groups based on the expression of immune checkpoints: the first group was positive for both iPD-1 and/or iPD-L1, and iCTLA-4 (n=58, 26.2%), the second was positive for only one of the two pathways (n=71, 32.1%), and the third group was negative for both pathways (n=92, 41.6%). Kaplan-Meier estimates showed that the difference between the overall survival curves of the three groups was marginally significant (P =0.060; Fig. 4). When further analyzed, a significant difference was observed for non-cancer-related survival (P =0.025), but not for cancer-specific survival (P =0.807; Fig. 4).
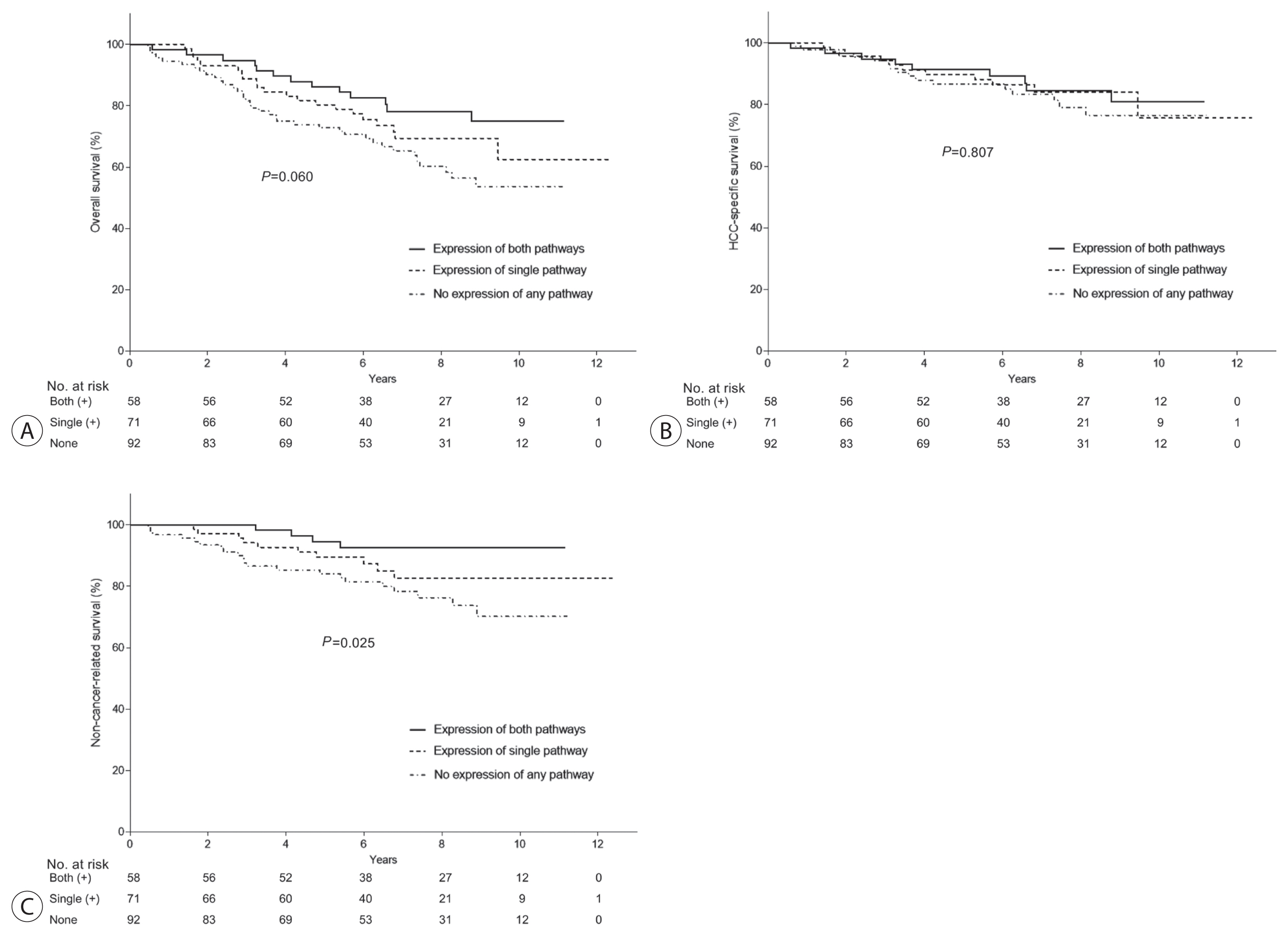
(A) Effect of combined expression of the PD-1/PD-L1 and CTLA-4 pathways on overall survival. The patients were divided into the following three groups based on the expression of immune checkpoint proteins: activation of both pathways (PD-1:PD-L1 & CTLA-4; group 1), activation of only one of the two pathways (group 2), and activation of neither pathway (group 3). The difference between the overall survival of the three groups was marginally significant (P=0.06). When further analyzed, a significant difference was observed for non-cancer-related survival (C), but not for cancer-specific survival (B). PD-1, programmed-cell death-1; PD-L1, programmed-cell death ligand-1; CTLA-4, cytotoxic T lymphocyte antigen-4.
DISCUSSION
Most HCCs arise on a background of chronic inflammatory liver, and thus are considered pathophysiologically typical immunogenic cancers.30–32 Based on the immunological mechanisms thought to be acting during hepatocarcinogenesis, the effects of diverse immunomodulatory regimens such as therapeutic vaccination, immune checkpoint inhibitors, and transfer of adoptive cellular immunity, have been investigated. 33–35 The efficacy of immune checkpoint blockers in patients with chronic liver disease and related HCC has been proved in randomized controlled trials, and approved for standard use in current care of advanced HCC.36,37 However, the efficacy of targeted immune checkpoints in patients with chronic liver disease and related HCC is unclear.
Previous studies have suggested that abnormal regulation of immune checkpoint proteins in tumors is linked to better or to worse prognosis depending on the type of cancer.13–15,18,19 Quantitative meta-analyses of data on solid tumors has suggested that overexpression of PD-L1 in tumor cells, or PD-1 in tumor infiltrating immune cells, is associated with poor prognosis in patients with malignant neoplasms of epithelial origin such as esophageal, gastric, colorectal, breast and ovarian cancers.13 This could be due to the ability of the PD-1/PD-L1 pathway to inhibit T cell-mediated antitumor immunity and to act as an anti-apoptotic receptor on cancer cells. On the other hand, tumoral expression of PD-L1, rather than PD-1, has been shown to have beneficial effects in patients with non-small cell lung cancer, metastatic urothelial carcinoma, colorectal cancer of the proficient mismatch repair type, and laryngeal cancers.14,19,38 It appears that CD8-positive T cells recruited from the tumor microenvironment induce a partial tumoricidal immune response, and promote upregulation of PD-L1 by secreting interferon-γ.39–41 This antitumor potential of the PD-1/PD-L1 axis was also observed in our HCC series. However French and Chinese studies of immune checkpoints in HCC have yielded inconsistent results. 21,22,25
The role of CTLA-4 signaling, another immune checkpoint pathway, in the clinical setting of malignancy has been little investigated, and there are conflicting results about its impact on outcomes in patients with different forms of cancer.17,28,29,42 Unlike a previous study on esophageal cancer,29 our findings indicate that positive CTLA-4 expression in lymphocytes is associated with a better prognosis in HCC, as it is in breast cancer:42 it seems that a high density of CTLA-4-positve lymphocytes is a secondary product of an increase of effector T cells that promotes immune invasion fighting against cancer.38,42,43 Curiously, CTLA-4 was not detected within tumor cells in our and others’ studies of HCC. In addition, in animal experiments, at least one third of the tumor-infiltrating lymphocytes expressed CTLA-4 along with PD-1, which might lead to synergistic immune modulation.44 Interestingly, co-expression of the two signaling pathways and their additive effect on survival was also noted in our study.
It is well known that while the CD8+ T cell-mediated immune response during infections by hepatitis B and C viruses contributes to viral clearance, it also has a cytotoxic effect on the host liver.9,12 Moreover, T cells also affect the progression of liver disease by inducing inhibitory immune checkpoint proteins.10,11 This immune dampening during the chronic phases of liver disease may have a protective effect by limiting excessive necroinflammatory and fibrogenic responses in the liver, so reducing the chance of cancer development.12 Such a process may account for our observation that immune checkpoint expression consistently reduced deaths unrelated to HCC rather than cancer-related deaths due to recurrence.
In our series, tumors expressing immune checkpoint proteins were more aggressive, with higher levels of AFP and greater immature and invasive pathology, which is in line with data from a prior French report.21 The invasive effect of tumor immune checkpoints has also been observed in other solid neoplasms such as melanoma, renal cell carcinoma and breast cancer.15,45,46 Despite the positive impact of the attenuation of hepatic inflammation by immune checkpoints, their injurious effect in interfering with antitumor immunity may justify blocking them as a therapeutic option in incurable HCC patients.
Our study has some limitations. The tissue microarray method used may not reflect the potential heterogeneity of immune checkpoint expression, although it can evaluate multiple samples rapidly. This weakness discouraged us from evaluating numbers of tumor infiltrating lymphocytes.47 Another consideration is that the roles played by immune checkpoint pathways in more advanced tumors could not be clarified in our surgical cohort. In addition, immune checkpoint signaling has been found to be upregulated under hypoxic conditions, and such conditions were often induced during treatment of the HCCs by chemoembolization or with sorafenib.48,49 Lastly, because most patients included in this study were infected by HBV, the prognostic role of immune checkpoint markers according to the etiology of HCC should be evaluated in future studies.
In conclusion, our investigation reveals the individual and additive effects of immune checkpoint molecules upregulated in the tumor-infiltrating mononuclear cells of HCC tissues in prolonging the survival time of patients, specifically survival not related to cancer recurrence. Because of the beneficial effects of immune checkpoints on survival, careful selection of therapeutic interventions might be required to avoid any harmful effects in HCC patients with indications for immunotherapy.
Notes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Ethics Statement
The study protocol was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2011-0931).
Funding Statement
This study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT (NRF-2017R1E1A1A01074298) and the research fund of Hanyang University (HY-201900000002619).
Data Availability
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Author Contribution
Data acquisition: HJK, EY
Data interpretation: JA, HJK, EY, HCL, JHS
Funding acquisition: JA, JHS
Statistical analysis: JA, JHS
Writing - original draft preparation: JA, HJK, JHS
Writing - review & editing: JA, HJK, EY, HCL, JHS
Approval of final manuscript: all authors
Supplementary Material
Supplementary data can be found with this article online https://doi.org/10.17998/jlc.2022.03.06.