Curative liver transplantation after lung resection for advanced hepatocellular carcinoma with lung metastasis and inferior vena cava tumor thrombosis: a case report
Article information
Abstract
Hepatocellular carcinoma (HCC) with distant metastasis is an absolute contraindication for liver transplantation (LT). However, it is still unclear whether LT is feasible or acceptable in such patients, albeit after being treated with a multidisciplinary approach and after any metastatic lesion is ruled out. We report one such successful treatment with living donor LT (LDLT) after completely controlling far-advanced HCC with inferior vena cava tumor thrombosis and multiple lung metastases. The patient has been doing well without HCC recurrence for eight years since LDLT. The current patient could be an anecdotal case, but provides a case for expanding LDLT indications in the context of advanced HCC and suchlike.
INTRODUCTION
Because of the high risk of hepatocellular carcinoma (HCC) recurrence after liver transplantation (LT), there is a need for strict selection criteria for performing LT in order to achieve an optimal prognosis. Once HCC recurs after LT, the outcome of the patients is quite dismal and the five-year survival rates are expected to be less than 20%.1 To avoid HCC recurrence after LT, careful patient selection is necessary. Conventionally, Milan criteria have been accepted as the gold standard to select LT for HCC, though several extended criteria such as University of San Francisco criteria, and up-to-seven criteria exist.2 However, we still do not have definite criteria to decide on LT for advanced HCC patients. Milan criteria would be too limited to give a chance to those patients who could get more survival benefits from LT. For this reason, many centers have tried to expand their selection criteria. 3
Despite these attempts, distant metastasis of HCC is considered to be an absolute contraindication for LT; LT cannot treat systemic metastasis and to date, there has been no report of LT in HCC patients with distant metastasis. Herein, we report a pioneering case of successful LT following multi-disciplinary down-staging treatment for a patient with advanced HCC with inferior vena cava (IVC) tumor thrombosis and lung metastasis before transplantation. This case report is described according to the CARE guidelines available from https://www.care-statement.org/.
CASE REPORT
A 50-year-old male was referred to our center for the treatment of a large HCC in the right lobe of the liver in April 2009. Pain in the right upper quadrant started a month before he visited a local hospital. His condition was diagnosed with hepatitis B virus 25 years earlier. He had been administered lamivudine since 36 years of age; however, he quit lamivudine 3 years prior to the referral. He had no history of alcohol abuse but a smoking history of 30 pack-years. The patient’s general condition was good. The laboratory findings were as follows: white blood cell count, 6,770/μL; hemoglobin, 17.7 g/dL; platelets, 142,000/μL; total bilirubin, 1.0 mg/dL; albumin, 4.1 g/dL; international normalized ratio, 1.22; aspartate aminotransferase, 94 IU/L; alanine aminotransferase, 65 IU/L; blood urea nitrogen, 12.1 mg/dL; and creatinine, 0.92 mg/dL. The alpha-fetoprotein (AFP) level was 9.62 ng/mL, and protein induced by vitamin K absence or antagonist-II (PIVKA-II) level was >2,000 pg/mL. Liver function was preserved (Child-Pugh score, A). Serological tests for hepatitis B surface antigen and antibody to the core antigen were positive, but were negative for the antibody against hepatitis C virus.
Initial contrast-enhanced computed tomography (CT) showed a large hypervascular mass in the right lobe with hepatic veins, IVC thrombosis, and multiple satellite nodules (Fig. 1). Positron emission tomography (PET)-CT showed a large mass with increased fluorodeoxyglucose (FDG) uptake in the right lobe of the liver, indicating high-grade HCC. Tumor thrombosis with FDG uptake in the IVC was observed (Fig. 2). There was bilateral pulmonary arterial thromboembolism with FDG uptake, which resulted in possible tumor thrombi. It revealed multifocal consolidations and ill-defined nodules in both lungs, which indicated infarction due to embolism.
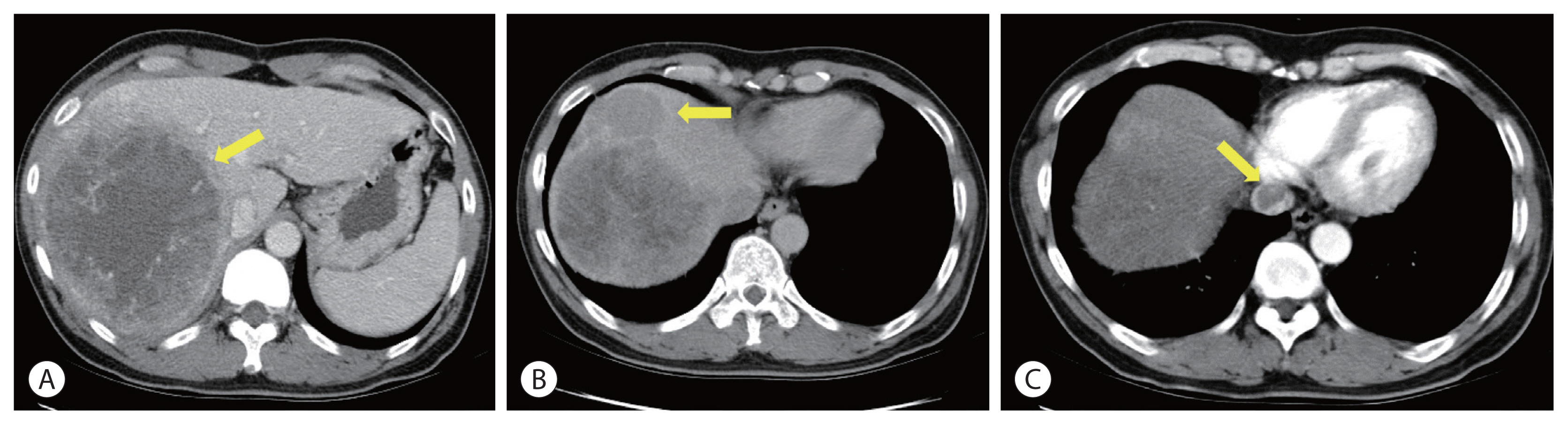
Initial computed tomography scan. A large hepatocellular carcinoma entirely occupied the right lobe (A), multiple satellite nodules (B) are seen. Tumor thrombosis is seen in the suprahepatic inferior vena cava (C) (arrows).
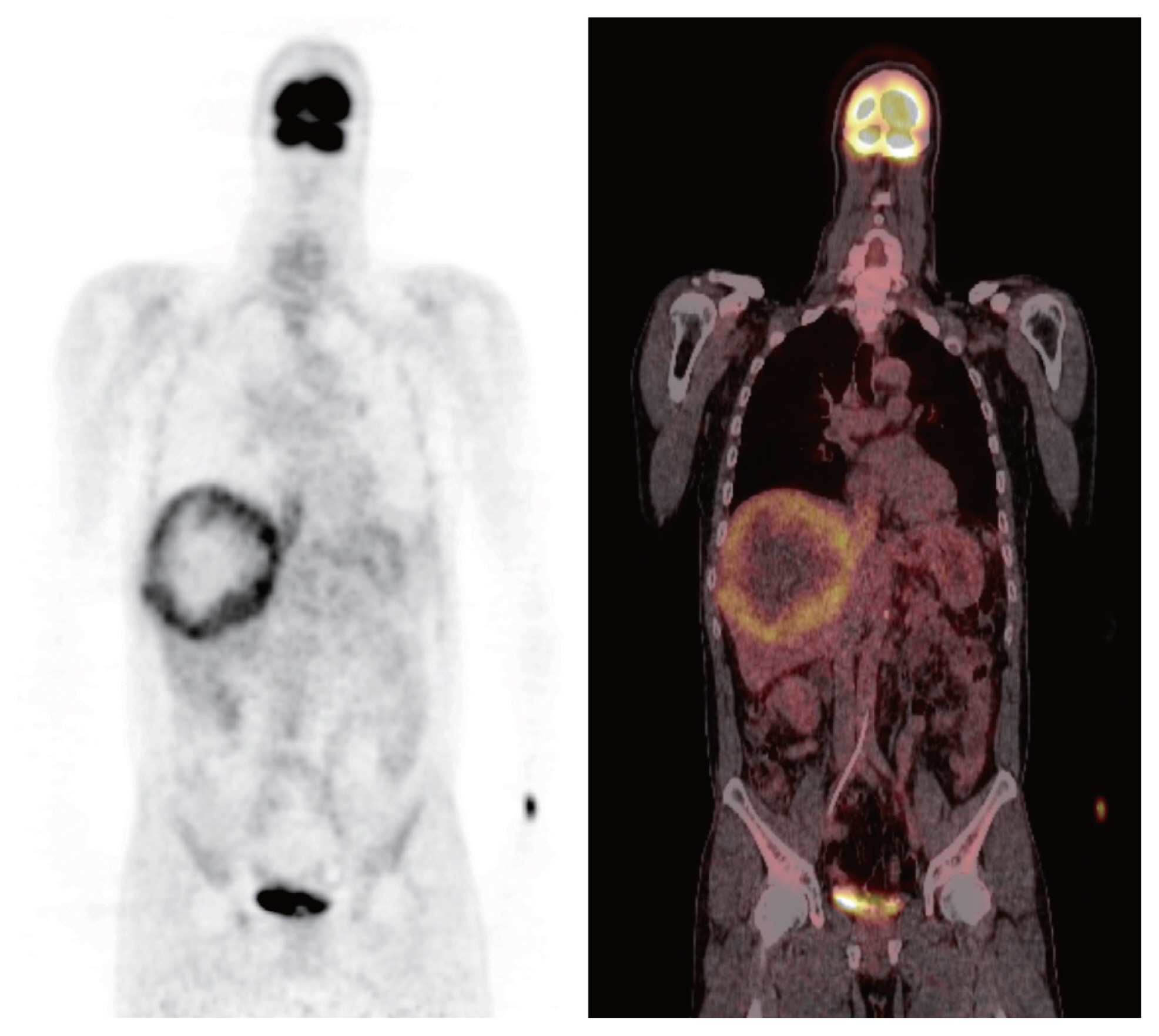
Initial positron emission tomography computed tomography scan. A large mass with increased fluorodeoxyglucose (FDG) uptake in the right lobe of the liver indicated high-grade hepatocellular carcinoma. Tumor thrombosis in the inferior vena cava shows FDG uptake.
Concurrent chemoradiotherapy (CCRT) followed by hepatic artery infusion chemotherapy (HAIC) was chosen as the first treatment after talks in a multidisciplinary conference. 4 Three-dimensional conformal radiotherapy was performed with 45 Gy in 25 fractions for 5 weeks. After CCRT, the primary tumor decreased and was near-totally necrotized. However, in the CT scan taken for response evaluation, there were newly developed multiple lung metastases, even though the primary liver tumors showed partial response to treatment (Fig. 3). We continued HAIC with 5-fluorouracil (at a dose of 500 mg/m2 for 5 hours on days 1 to 3) and cisplatin (at 60 mg/m2 for 2 hours on day 2). After 15 cycles of HAIC treatment, all hematogenous lung metastatic lesions, except a single lesion on the right upper lung, disappeared. The residual single metastatic nodule showed an increased size (Fig. 4A). At the multidisciplinary conference, we decided to perform lung wedge resection to remove residual nodules in the lung. Video-assisted thoracoscopic (VATS) wedge resection was performed. The histological findings of the lesion showed poorly differentiated carcinoma, favoring metastatic HCC (Fig. 4B).
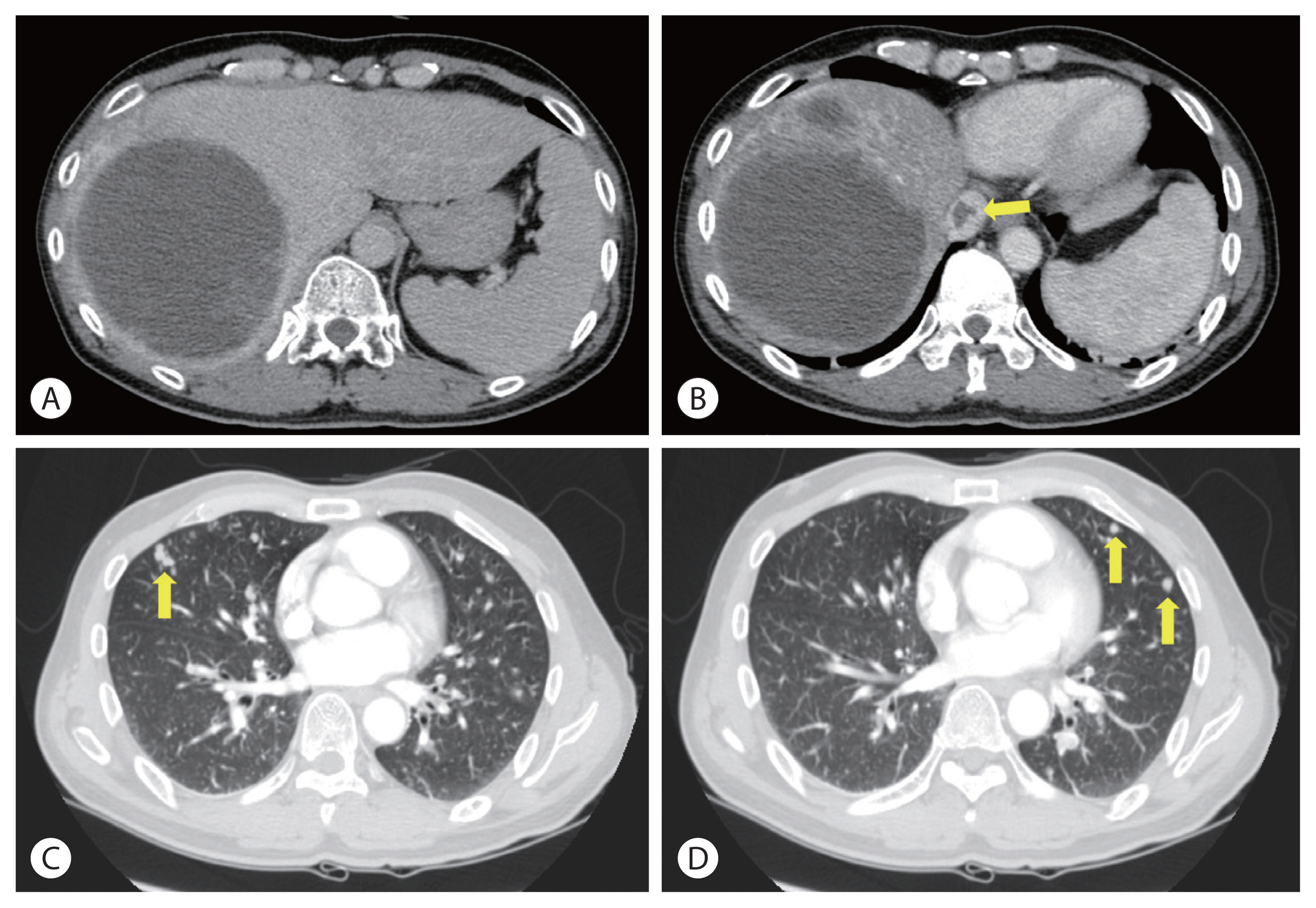
Liver and chest computed tomography scan after concurrent chemoradiotherapy. The primary tumor decreases and is near-totally necrotized (A). Inferior vena cava thrombosis reduces (B). However, multiple metastatic nodules are seen in both lung fields (C, D) (arrows).

Chest computed tomography scan after 15 cycles of hepatic artery infusion chemotherapy. All the hematogenous lung metastatic lesions except a single lesion on the right upper lung disappear. The residual single metastatic nodule shows increased size (A, arrow). The histological findings show poorly differentiated carcinoma, favoring metastatic hepatocellular carcinoma (B, arrows indicate the tumor margin) (hematoxylin and eosin, ×40).
Even after lung resection, HCC recurred repeatedly in other parts of the liver. We performed radiofrequency ablation (RFA) for a 2.6 cm-sized tumor on segment 2, 1 month after VATS. Transarterial chemoembolization (TACE) was performed for three recurrent lesions on segment 6, 6 months after the RFA. Finally, another RFA treatment was applied to segment 2, 10 months after TACE.
After completing the above treatments for recurrent lesions in the liver, there were no more viable tumors in imaging studies, including CT and PET-CT scans. However, liver function of the patient gradually worsened. His platelet count decreased to 22,000/μL, and his total bilirubin level was elevated to 2.6 mg/dL. After extensive internal discussions on whether LT could be beneficial in this scenario, we decided to wait for more than 3 months to define the tumor characteristics before progressing with LT. The patient and his family members were very favorable toward LT and were willing to take the risk of HCC recurrence after LT. Meanwhile, we evaluated his son as a live donor. As there was no recurrence during the waiting period, we planned a living donor LT (LDLT). This LDLT procedure was accomplished 4 months after the last locoregional treatment, which was almost 4 years after the initial diagnosis.
The patient underwent LDLT with a modified right lobe graft in January 2013. At the time of LDLT, his AFP and PIVKA-II tumor markers were 65.68 ng/mL in AFP and 32 mAU/mL, respectively. He recovered uneventfully with standard postoperative care and immunosuppression. The explanted liver showed four HCCs that were near-totally necrotized, with no microvascular invasion (Fig. 5).
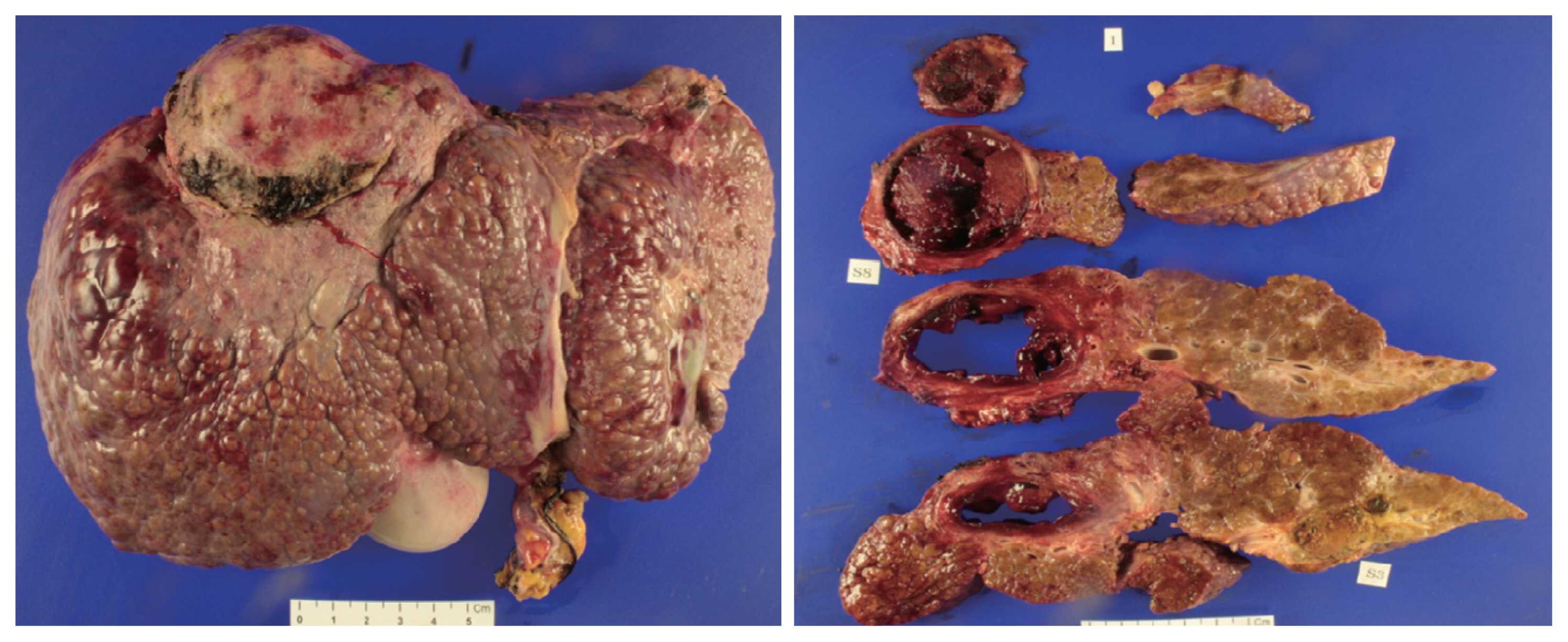
Explanted liver. The explanted liver shows four hepatocellular carcinoma tumors that were totally necrotized with no microvascular invasion. The largest tumor is 6×5.5 cm in size (on segment 5); there are no viable lesions.
We started his maintenance immunosuppression with tacrolimus and low-dose steroid and substituted tacrolimus with the mammarian target of rapamycin (mTOR) inhibitor, sirolimus 3 months after LT. Since then, he has been administered sirolimus monotherapy for immunosuppression without calcineurin inhibitors and steroids. It has been 8 years since the patient underwent LDLT. There has been no HCC recurrence so far. He is doing well, without experiencing any complications related to LT or immunosuppression.
DISCUSSION
As HCC primarily arises from chronic liver disease, LT replacing the native liver has been established as the standard treatment in selected patients for the last two decades.5 However, applying LT to far-advanced HCC is still controversial because the risk of recurrence of these tumors is very high, and a good result may not be expected. However, many centers have attempted to expand the selection criteria for HCC patients in order for them to undergo LT. It seems that the Milan criteria would not be the Bible anymore.3,6–8 Nevertheless, distant metastasis of HCC is still considered an absolute contraindication for LT. There is no literature on successful LT performed on a patient with metastatic HCC. Since 2012, our center has been trying to expand LT criteria for advanced HCC through a multidisciplinary approach. When the down-staging for those patients succeeds, we carefully select them to undergo LT. We reported an acceptable result of our initial experience with curative LT for patients who had portal vein tumor thrombosis after successful down-staging with CCRT.9 In this study, we reported that five patients out of eight had reached the 3-year mark of recurrence-free survival even though they were considered as a contraindication for LT, and their life expectancy would have been less than 1 year without LT. From this early experience, we learned that careful selection could show good outcomes even for patients with far-advanced HCC with portal vein tumor thrombosis who showed good response to pretransplant locoregional treatment, including CCRT. We selected one such patient, carefully considering the tumor biology and observing the disease progress. We discussed all possible outcomes with his family members, and obtained informed consent. All family members were eager for LT, and they were willing to donate their liver to the patient. Due to organ shortage problem, to avoid futile transplantation, strict selection criteria for patients with HCC have been considered in deceased donor liver transplants. But, LDLT has different story because the graft should be used only for the family member. Thus, we could cautiously expand LT criteria for those with advanced HCC to include those who did not show any disease progression after down-staging therapy as long as the patient and family members understand all the circumstances and are willing to risk HCC recurrence in the absence of other options. Disease progression even after locoregional or systemic therapy before LT can be one of the independent risk factors for HCC recurrence after LT, implying aggressive tumor characteristics.10 Thus, we waited at least 3 months after the last RFA to perform LT and checked if there was no viable tumor in the liver and lung just before the transplantation. This patient had not shown any disease progression for over 3 months.
As HCC treatment should vary according to tumor size, number, location, and aggressiveness, a multidisciplinary approach is necessary to determine treatment modalities. Our center has regularly discussed complicated HCC cases at multidisciplinary conferences that include hepatologists, radiation oncologists, liver surgeons, medical oncologists, interventional radiologists, and nuclear medicine specialists. This led us to have established our unique treatment for advanced HCC, similar to the current case, which is CCRT followed by HAIC.4 Utilizing this protocol, we have shown promising results, making the unresectable or non-transplantable patients undergo curative surgery such as liver resection or transplantation.9,11 We believe the patient, in this case, gained the absolute benefit of multidisciplinary treatment. He was a good responder to CCRT and systemic chemotherapy, which made him undergo curative surgery.
In conclusion, we cannot generalize this case for all patients with distant metastasis of HCC. This patient might have been an anecdotal case. However, this report suggests that metastatic HCC should not be an absolute contraindication if the patient is a good responder to combined locoregional and systemic therapy, and if there is no more viable lesion at the time of the LT procedure.
Notes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Ethics Statement
This case report was approved by the Ethical Committee and Institutional Review Board of Severance Hospital (IRB No.4-2021-1001). The operation was performed after obtaining informed consent from the patient and his family members.
Funding Statement
No funding to declare.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed for this case report.
Author Contribution
Conceptualization: DJJ
Data curation: DJJ, HJK
Methodology: DYK, JS, JGL
Project administration: DJJ, JGL
Writing-original draft: DJJ
Writing-review & editing: DJJ, DYK, JS, JGL, DHH, MSK, HJK, JSC, SIK
Approval of final manuscript: all authors
