Rare Case of Pyogenic Brain Abscess after Transarterial Chemoembolization in a Patient with Hepatocellular Carcinoma: Case Report and Literature Review
Article information
Abstract
Transarterial chemoembolization (TACE) is a useful treatment option for hepatocellular carcinoma (HCC). TACE can particularly be used as a treatment for localized HCC, where surgical resection is impossible due to decreased liver function. However, TACE is associated with several complications, including vascular complications, liver failure, non-target embolization, infection, and death. The main risk factor for complications after TACE is decreased liver function. There have been only few reports of brain abscesses after TACE that are difficult to be distinguished from hepatic encephalopathy. Here, we report a rare case of brain abscess caused by Klebsiella pneumoniae that occurred after TACE.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a major cancer type in Korea. Chronic hepatitis B has been the most common cause of HCC in Korea, and the incidence of HCC is still increasing due to prevalence of fatty liver conditions in the general population.1,2 In Korea, transarterial chemoembolization (TACE) is frequently performed as the initial treatment (41.7% cases).3 TACE is recommended as a standard treatment for intermediate-stage HCC, and is also clinically administered in some patients with advanced HCC and single nodular HCC with reduced liver function. However, as TACE may initiate various complications, the patient must be evaluated before and followed closely after the procedure. The major complications include blood vessel injury, liver failure, nontarget embolization, infection, and death.4 Clinically, abscesses are often observed after TACE, but they are mostly confined to intrahepatic lesions. In theory, if such an abscess is not well-treated and develops into sepsis, it can further lead to an extrahepatic abscess. However, these distant organ abscesses are very rare.4 Moreover, abscesses occurring in the brain are extremely rare, and only one case of brain abscess after TACE has been reported.5 Here, we report a rare case of brain abscess caused by Klebsiella pneumoniae occurring after TACE.
The present case report was approved by the Institutional Review Board (IRB) of the Soonchunhyang University College of Medicine, Bucheon, Korea (IRB number: SCHBC 2020-12-011). The study protocol conformed to the ethical guidelines of the Declaration of Helsinki of the World Medical Association.
CASE REPORT
1. Clinical findings
A 60-year-old woman visited the outpatient clinic with symptoms of fever, neck stiffness, motor weakness, inappropriate language, and disorientation. She had undergone TACE for HCC 12-days earlier and was discharged after three days without complications. The HCC tumor was small sized, single nodule of 1 cm, but her liver function deteriorated to Child-Pugh class B (8–9); thus, TACE was performed. The HCC image findings are shown in Fig. 1, and TACE image findings are shown in Fig. 2.
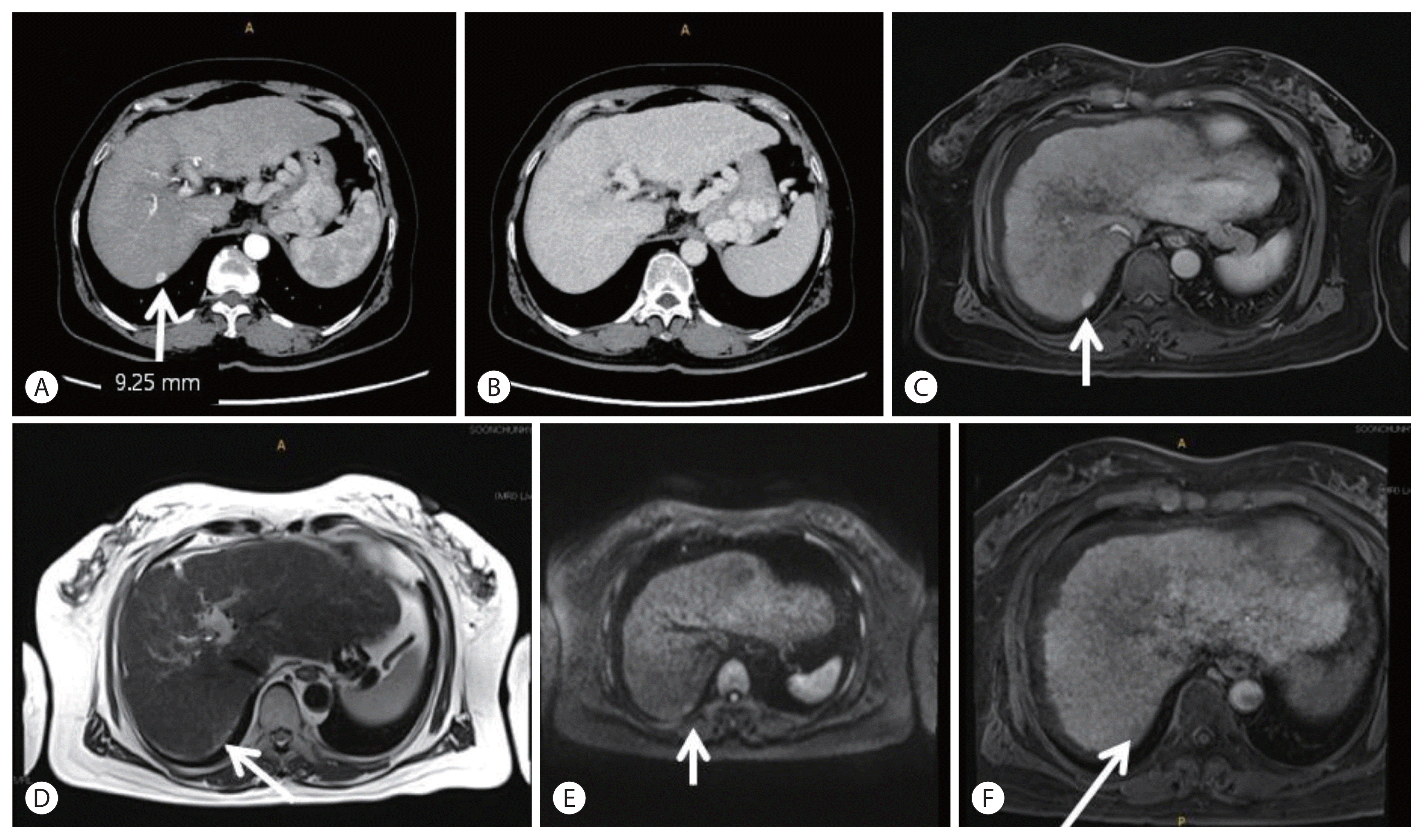
Imaging findings of hepatocellular carcinoma (HCC). (A) A focal 9 mm nodular arterial-enhancing lesion is visible in the subcapsular portion of the right posterior liver (white arrow; liver computed tomography [CT], arterial phase). (B) The lesion is not washed out in the delayed phase. Other imaging studies, such as liver magnetic resonance imaging (MRI), are required (liver CT, delayed phase). (C) Additional liver MRI showed arterial enhancement with increased size of lesion (white arrow; liver MRI, T1WI with Primovist enhancement). (D) The lesion showed a subtle increase in signal intensity on T2-weighted imaging (white arrow; liver MRI, T2WI). (E) In the diffusion-weighted image, the signal intensity increased subtly (white arrow; liver MRI, diffusion weighted image [DWI]). (F) Signal defects in the hepatobiliary phase (HBP) are observed, suggesting the possibility of early HCC (white arrow; liver MRI, T1WI with contrast-enhanced, 20 minutes).
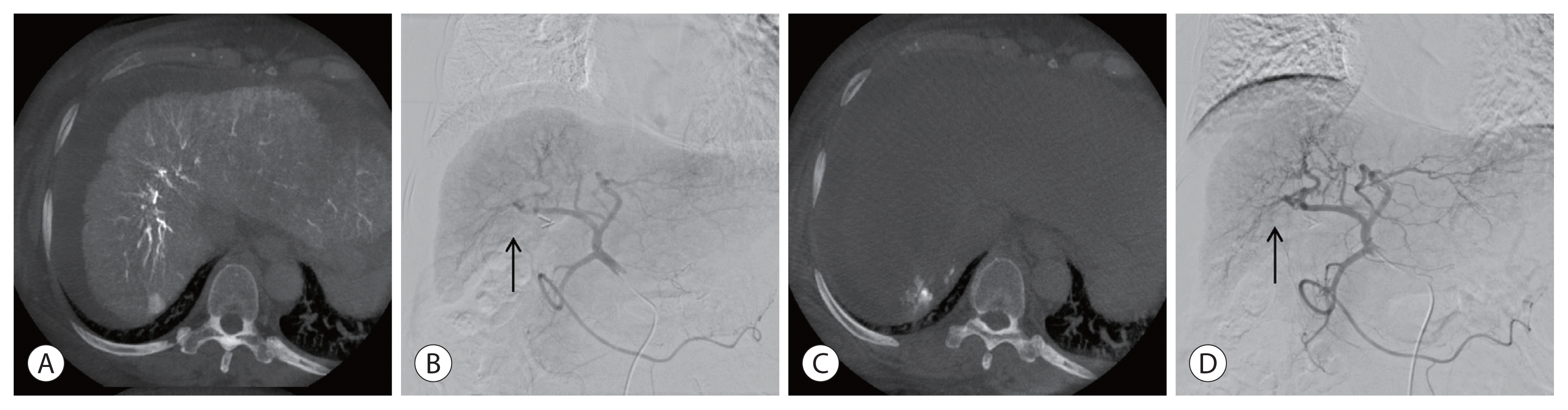
Imaging findings of transarterial chemoembolization. (A, B) A small tumor staining (arrow) in the right lobe. (C) Embolization is performed via feeding branches of the right hepatic artery. (D) Postangiography shows no residual tumor staining (arrow).
She was previously diagnosed with liver cirrhosis and chronic hepatitis B in 1996 and has been administering tenofovir since then. In 2010, she was diagnosed with type 2 diabetes mellitus and was being treated at another hospital. In September 2010, she underwent surgery at another hospital for ventral hernia, but no ascites were observed at that time. In August 2019, she was hospitalized for surgery for septic arthritis of the left elbow joint. At that time, preoperative evaluation was performed before administering general anesthesia, and decompensated cirrhosis and portal hypertension were diagnosed. Additionally, abdominal ultrasound imaging showed substantial ascites, and transient elastography showed a pressure of 75 kPa. In the blood test, hepatitis B virus (HBV) e-antigen (HBeAg) was negative, but antibody for HBV e-antigen (anti-HBe) was positive, HBV DNA titer was 165.7 IU/mL, and alpha fetoprotein level was 2.8 ng/mL. Gastroduodenoscopy revealed gastric varix (type GOV2) and grade II esophageal varix. She was receiving dapagliflozin 10 mg/day, sitagliptin 100 mg/day, metformin 1 g/day, and amaryl 4 mg/day as diabetes medication, but her glycated hemoglobin (HbA1c) level was 10.0, resulting in poor diabetes control. In September 2019, an alteration of mental status occurred, and she was admitted to the emergency room and diagnosed with hepatic encephalopathy. Oral diuretics were subsequently used to control ascites at the outpatient clinic.
The patient’s initial vital signs were as follows: blood pressure, 98/55 mmHg; pulse, 80 times/min; body temperature, 37.5°C; and respiratory rate, 20 breaths/min. On neurological examination, mental state was alert, but she had slurred speech. Generalized motor weakness was found in all four limbs (motor grade IV), and she had slight neck stiffness.
The results of the initial blood test at the hospital showed that the white blood cell count was 8,350/mm3, total bilirubin 4.54 mg/dL, direct bilirubin 1.39 mg/dL, aspartate aminotransferase (AST) 101 IU/L, alanine aminotransferase (ALT) 84 IU/L, alkaline phosphatase 229 (normal 30–120) IU/L, gamma-glutamyl transferase 319 IU/L, prothrombin time (PT) international normalized ratio (INR) 1.3 (87%), and high sensitivity C-reactive protein (hs-CRP) 0.80 IU/L. Moreover, Child-Pugh score was 8 (Child-Pugh class B) and model for end-stage liver disease (MELD) score was 15 points.
Arrhythmia was absent before admission, although paroxysmal atrial fibrillation developed during the ICU care period. Echocardiography and 24 hours Holter monitoring were performed, but we could not find evidences for any other causes that resulted in brain abscess.
2. Imaging findings
The patient was initially diagnosed with hepatic encephalopathy after TACE, but due to the accompanying fever and neck stiffness, additional brain imaging tests were performed. Brain computed tomography (CT) and magnetic resonance imaging (MRI) showed a 3.2×3.3 cm well-defined, hypoattenuating lesion with perilesional edema in the left basal ganglion, left periventricular white matter, and posterior limb of the internal capsule. Imaging findings of the brain abscess are shown in Fig. 3.
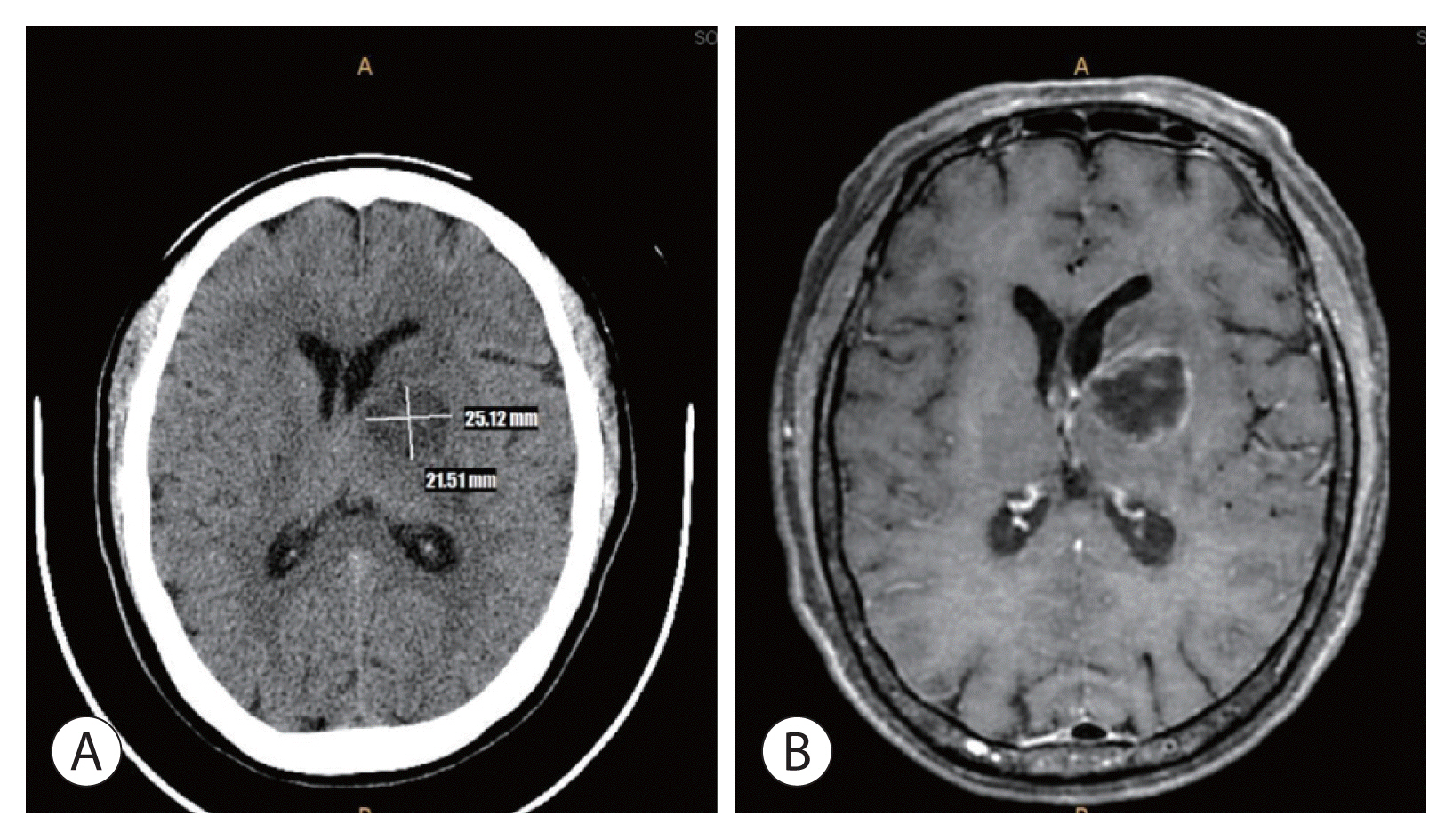
Imaging findings of the brain abscess. Brain computed tomography (A) and magnetic resonance imaging (B) show an approximately 3.2×3.3 cm well-defined hypoattenuated lesion with perilesional edema in the left basal ganglia, left periventricular white matter, and posterior limb of the internal capsule, along with brain abscess.
3. Diagnosis and treatment
Based on the clinical symptoms and imaging tests, the patient was diagnosed with a brain abscess developed after TACE. Blood culture was immediately performed, and meropenem, vancomycin, and metronidazole were administered as empirical antibiotics. However, the neurological symptoms worsened, and navigation-guided drainage of the brain abscess was performed one day after hospitalization. Klebsiella pneumoniae was identified in both the blood culture and culture of pus drained from the brain abscess. Antibiotics were changed to ceftriaxone-alone as the blood and pus cultures showed extended spectrum beta-lactamases (ESBL)-negative K. pneumoniae. Abdominal CT performed to check for intrahepatic abscess showed only tumor necrosis due to TACE, with no evidence of liver abscess.
One week after brain drainage, the patient’s neurological symptoms improved, and the motor grade recovered to grade IV. After surgery, fever also reduced, and antibiotic treatment was maintained. However, fever and dyspnea developed 17 days after surgery. Pneumonia was detected on chest radiographs. As the patient developed hospital-acquired pneumonia, the antibiotics were changed to meropenem-alone, and a tracheostomy was performed. However, the pneumonia gradually worsened, and hepatorenal syndrome developed on the 24th day after surgery. The patient died 32 days after surgery due to uncontrolled infection, and worsening of liver and kidney function.
DISCUSSION
Brain abscesses rarely occur in patients with head and neck trauma and immunocompromised conditions, such as acquired immunodeficiency syndrome (AIDS), or the use of glucocorticoids.6 In Western population, the incidence rate of brain abscess is approximately 1,500–2,500 cases per year.7 As brain abscess can be very fatal, immediate and intensive treatment is required. Therefore, it is necessary to implement both surgical and antibiotic treatments. The causative pathogens differ depending on the cause of brain abscess. When it is spread from the otitis media, Bacteroides, Peptostreptococcus, and Streptococcus are the main causative bacteria; and in case of heart disease, especially cyanotic heart disease, Peptostreptococcus, and Streptococcus, mainly Streptococcus viridans and microaerophiles, are the main causative bacteria.8 Staphylococci and Streptococcus are the main causative bacteria in brain abscesses after brain surgery.9 Staphylococcus, Streptococcus, Clostridia, and Enterobacteriaceae are the causative bacteria in brain abscesses due to infection during head trauma. In recent years, an increase in the number of immunosuppressed patients with HIV infection, chemotherapy for cancer, and organ transplantation has led to diversification of the causative pathogens of brain abscesses from bacteria to fungi.8,9 Cases with fungi, Toxoplasma, Staphylococcus spp, Streptococcus spp, and Pseudomonas spp infections that were rare before, are increasing.10
TACE is an effective treatment for patients with intermediate stage of HCC, but it can lead to various complications. For instance, there are rare reports of extrahepatic abscesses, including to the spleen and lung, and subsplenic space abscesses and epidural abscesses.11–14 Moreover, brain abscesses are extremely rare.5,14 Distant organ abscesses after TACE may be mainly due to bacteremia arising from liver abscess. Ischemic damage and bile duct damage during TACE can lead to infection, liver abscesses, bacteremia, and extrahepatic abscesses.15
In the present case, the primary HCC tumor was small sized, and thus, it failed to form liver abscess; but, bacteremia that occurred during necrosis of the tumor may have caused the brain abscess. Klebsiella pneumoniae, an uncommon cause of brain abscess, was identified as the causative pathogen, supporting the occurrence of temporary bacteremia due to damage to the liver and biliary tract tissue after TACE.
To date, there is only one reported case of brain abscess after TACE.5 Similar to the previous case, our patient also showed brain abscess after TACE, with fever as a temporary symptom. However, fever improved at the time of diagnosis that identified Klebsiella pneumoniae as the causative agent. However, there are some differences between the previous and our case. In the previous case, brain abscess occurred three-months after TACE, with headache, loss of consciousness, and muscle weakness in the left upper limb as the symptoms. In contrast, in our case, the symptoms appeared 12-days after the first TACE, with improper and confused language as the primary symptom rather than those observed in grade IV disease. Table 1 summarizes the commonalities and differences between the previous and our case. A study on whether prophylactic antibiotics can reduce brain abscesses after the procedure suggested that they were not beneficial in patients with normal sphincters of Oddi.16
Clinically, a brain abscess occurring after TACE is difficult to be differentiated from hepatic encephalopathy. In our case, symptoms similar to hepatic encephalopathy were observed, such as improper language and mental disorders. Hepatic encephalopathy is a relatively common complication due to a temporary decrease in liver function after TACE. It often recovers well after symptomatic treatment. However, as brain abscess can cause serious neurological complications, clinical suspicion and early differential diagnosis of brain abscess are necessary. Brain abscesses, unlike hepatic encephalopathy, may accompany local neurological abnormalities; therefore, neurological tests for motor and sensory effects should not be neglected. Moreover, when fever or increased intracranial pressure is observed with neck stiffness or a positive Kernig sign, or if the symptoms progress slowly, a brain imaging study should be performed considering the possibility of brain abscess or brain metastasis.
In conclusion, although rare, brain abscess may occur after TACE, and clinical differentiation from hepatic encephalopathy may be necessary. The causative pathogen of brain abscess after TACE in this case was a gram-negative organism, unlike the general causative organisms. Surgical drainage and antibiotic therapy are recommended for brain abscesses, but the prognosis is poor.
Notes
FINANCIAL SUPPORT
This work was supported by Soonchunhyang university research fund.
Conflicts of Interest
All authors have no conflict of interest relevant to this study.
