Contrast-enhanced Ultrasonography: The Third Modality for Differentiation of Liver Mass
Article information
Abstract
Contrast-enhanced ultrasonography (CEUS) using microbubble ultrasonography contrast agent can show the vascular structure and unique contrast enhancement patterns of focal liver lesions, including hepatocellular carcinoma (HCC). CEUS shows three phases, similar to a vascular pattern on computer tomography (CT), and typical arterial enhancement and portal or late phase washout in HCC. CEUS can show real-time images without nephrotoxicity or radiation hazard and can be used as guidance for loco-regional treatment and estimation of treatment response of HCC. In addition, some data recently revealed the usefulness of CEUS in the early estimation of response to anti-cancer pharmacological (i.e., sorafenib) therapy in advanced HCC. Although CEUS has limitations in clinical practice and more investigation is needed for its validation, it is recommended as a main diagnostic modality in a few major clinical practice guidelines for HCC. Thus, greater understanding of CEUS is necessary to extend its application in real practice for diagnosis and management of diseases.
INTRODUCTION
Ultrasonography is widely used for diagnosis in various fields because it has no exposure to radiation, has high stability, and is easy to examine. However, conventional grey scale ultrasonography has limitations in that it cannot evaluate the vascular pattern of focal lesions. Contrast-enhanced ultrasonography (CEUS) using microbubble ultrasonography contrast agent (UCA) has a higher diagnostic power with various contrast patterns because it spreads to blood vessels and organ tissues in real time. In particular, the application of a second-generation UCA, SonoVue® (Bracco SpA, Milan, Italy), since mid-2000s improved the diagnostic accuracy in the diagnosis of hepatocellular carcinoma (HCC), and its use is also helpful in cases where ultrasonography-guided procedure is required. CEUS has a good ability to distinguish focal small liver lesion, although it has not been widely used in clinical practice; thus, further studies regarding its usefulness are needed to expand its clinical applications. This review will overview the usefulness of CEUS in clinical practice.
1. UCA
The second-generation microbubble UCAs available in Korea are SonoVue® and Sonazoid® (Daiichi Sankyo, Tokyo, Japan). These are all composed of gas with phospholipid coating, and they function based on the phenomenon of microbubbles resonating or collapsing to produce strong reflection on the ultrasonic wave [1]. The lipid membranes surrounding these gases differ depending on the UCA. The microbubbles are approximately 2-10 μm in size, which is similar to the size of red blood cells. They pass through the capillaries; hence, even if they are injected into the peripheral vein, they can circulate through the whole body, from the right heart to the lungs and then to the left heart before reaching the target organ [2].
CEUS shows three vascular phases, namely the arterial phase (from 10-20 to 30-45 [seconds]), portal venous (PV) phase (from 30-45 to 120 [seconds]), and late phase (from 120 seconds to bubble disappearance), because of the dual blood supply to the liver from the hepatic artery and portal vein [3,4]. The lesion is distinguished based on characteristic ultrasonographic images of each of these phases. The arterial phase is useful for finding the focal liver lesion. The late phase is useful for identifying malignant lesions that are of hypoechoic echo-pattern while the most benign lesions present iso-or hyperechoic features. In the case of malignant lesions such as HCC, the late phase shows a pattern with similar to that observed using dynamic liver computer tomography (CT), which is the washout of dynamic enhancement. The characteristics of SonoVue® and Sonazoid® UCAs are briefly described below.
1) SonoVue®
It was first developed and marketed in 2001. It is characterized by microscopic bubbles containing sulfur hexafluoride (SF6) gas, which is not soluble in water and blood, in a phospholipidic monolayer shell of 2-10 μm in size. SonoVue ® is helpful for evaluating the characteristics of blood vessels because it is less phagocytosed by cells and is mainly present in large and small blood vessels. In particular, SonoVue ® has good flexibility and resistance of micro-bubble shells to ultrasonic waves; thus, it can be used stably in various frequency ultrasonic waves. Moreover, it can be used in both microbubble vibration and disruption depending on the mechanical index (MI) [5].
2) Sonazoid®
Sonazoid®, which until 2011 was only used in Japan, was launched in Korea in 2012, and its use is gradually expanding [5]. Sonazoid® contains perflubutane gas in a lipid shell, is similar in size to SonoVue, and can be observed at low MI values (0.1-0.2). However, the echo pattern of Sonazoid® has unique features that can be categorized into two major stages: a vascular phase (arterial phase [to 30 seconds] PV phase [to 120 seconds], and late phase) and a Kupffer phase (10-15 minutes). This occurs because unlike SonoVue®, Sonazoid® is phagocytosed by Kupffer cells in the liver parenchyma approximately 10 minutes after its administration. In the absence of normal Kupffer cells, as in the case of HCC, such a phagocytic effect is not present, and it appears as a black defect without enhancement. Therefore, a late vascular phase (Kupffer phase) of CEUS is similar to the hepatobiliary phase of Gd-EOB-DTPA MRI, which is a useful advantage of Sonazoid® CEUS [6].
3) Safety
UCAs have an advantage as they are not renally toxic and can be safely used even in patients with impaired renal function and when CT examination is available [7]. UCAs also have limited side effects; i.e., the allergy or hypersensitivity to contrast is very low and life-threatening anaphylactic reactions occur at 0.001% [8]. In addition, unlike CT, there is no risk of radiation exposure even in repetitive examinations [9-11]. However, caution is required when using UCAs in patients with coronary artery disease and extracorporeal shock wave lithotripsy. Furthermore, UCASs are not recommended for use in pregnant or lactating women and in patients with severe pulmonary hypertension [12].
2. Clinical use of CEUS
CEUS has been used most actively in the diagnosis and treatment of liver disease. In particular, it is most commonly used to identify focal lesions in the liver and also as a guide to increase the accuracy and success rates in the biopsy or locoregional treatment; moreover, it is used to assess treatment response.
Focal lesions of the liver can be categorized into malignant lesions represented by HCC and benign lesions represented by hemangiomas and adenomas. The characteristics of typical benign lesions are that they exhibit hypervascularity in the arterial phase and similar echo to surrounding liver tissues in the late vascular phase. Malignant lesions also show hypervascularity in the arterial phase but washout in the late vascular phase. Table 1 summarizes the characteristics of each lesion on CEUS.
1) Diagnosis of HCC using CEUS
HCC is a highly vascular lesion, and dynamic CT and magnetic resonance imaging (MRI) are the main modalities used for its diagnosis. However, because CT and MRI are performed at a predetermined point in time, false-negative results may be obtained in HCC cases that show hyperacute enhancement in the arterial phase. In comparison, CEUS has the advantage of being able to observe enhancement patterns continuously on the patient's side in real time, and therefore, a more accurate diagnosis is possible in some cases. Ultrasonographic findings of HCC vary depending on the cellular differentiation, degree of fatty degeneration, and degree of necrosis and fibrosis of the tumor. CEUS is particularly useful for evaluating the characteristics of HCC and is rich in neovascularization because it can observe changes in enhancement in real time. Because typical HCC receives most of the blood flow from the neovascular artery, it is characterized by hypervascularity of the tumor as arteriolar enhancement and a washout with a hypoechoic appearance after portal phase owing to a decrease in portal blood flow as the neovascularization becomes more abundant. These remain the most important features for the diagnosis of HCC. However, caution is needed in some cases of non-typical HCC may not show hypervascularity in the arterial phase. The enhancement pattern of HCC is also related to the differentiation of carcinoma. Early HCC and well-differentiated HCC often have weak arterial phase enhancement and the degree of contrast in the late phase is similar to that of the surrounding normal tissue; hence, patients with a risk factor of HCC may require more attention [13].
The washout of HCC is slower and the degree of enhancement reduction is smaller than those of metastatic liver cancer, which may help in differentiating between HCC and metastatic liver cancer. Therefore, to differentiate other types of liver cancer, it is necessary to observe whether there is early washout in 1 minute and marked washout of punch-out pattern (Fig. 1) [14].
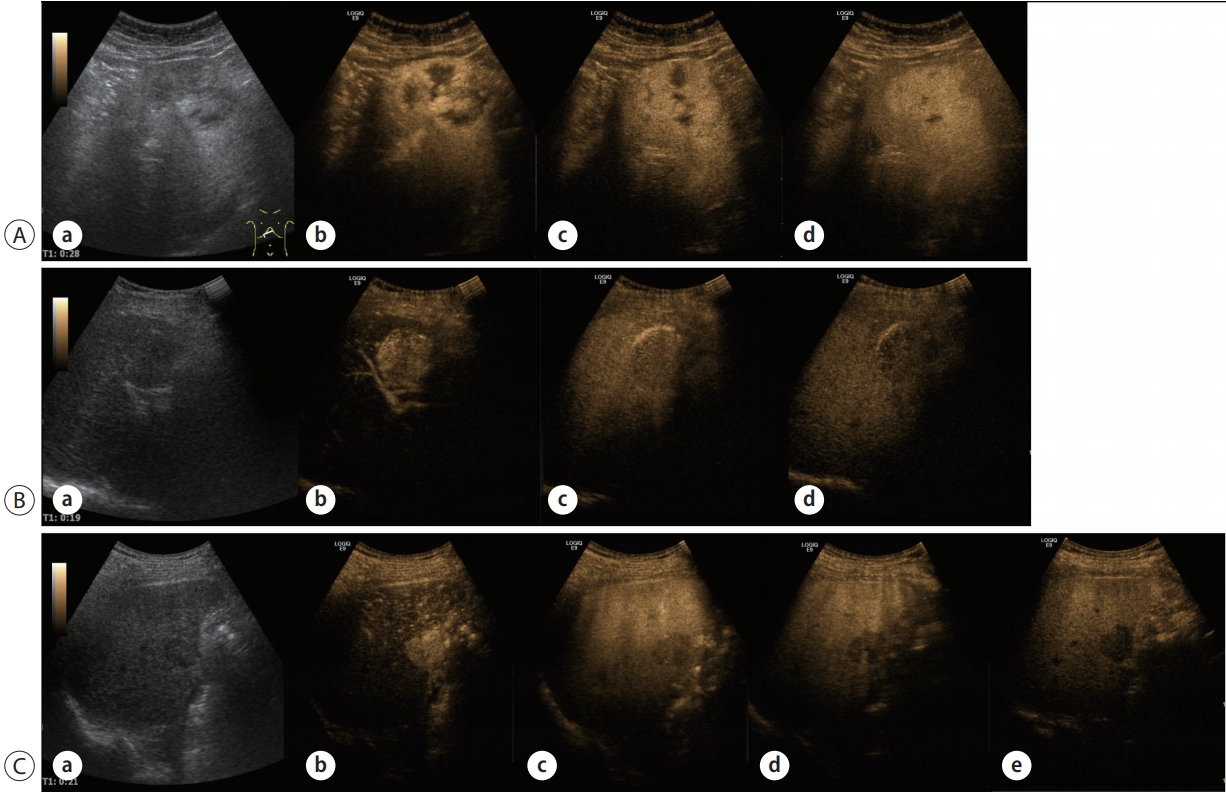
Example of enhancement pattern of hemangioma using SonoVue® (A), Hepatocellular carcinoma (HCC) using SonoVue® (B), and HCC using Sonazoid® (C) in contrast-enhanced ultrasonography (CEUS). Grey scale ultrasonography image of HCC (a). In general, compared with surrounding parenchyma, HCC shows hyperenhancement in the arterial phase (b) and washout in the portal (c) or late phase (d). In particular, Sonazoid® CEUS presents HCC as dark defects in the Kupffer phase (C, e).
3. CEUS in clinical practice guideline for HCC
CEUS has some weaknesses such as a relatively lower detection rate for washout of HCCs than CT or MRI [15-17], limitation in stage estimation (limited number of targeted observations per examination) [15,16], and limited performance in poor echo window. Therefore, most guidelines recommend CEUS as a secondary imaging modality. The European Association for the Study of the Liver (EASL) [18] does not strongly recommend the use of CEUS for the diagnosis of HCC, whereas the Korean Liver Cancer Association-National Cancer Center (KLCA-NCC) [19] and the Asian Pacific Association for the Study of the Liver [20] recommend CEUS as a second-line modality when initial first-line diagnostic imaging is not sufficient. In contrast, AASLD does not recommend CEUS as a diagnostic imaging for HCC because of the absence of a large-scale study, potential selection bias for patients with adequate quality ultrasonography, lack of generalizability of studies in Asia versus Western countries, and operator dependency.
Both EASL and KLCA-NCC guidelines in 2018 recommended the use of SonoVue® as a UCA for the diagnosis of HCC. However, both guidelines suggest the lesion larger than 1 cm should manifest arterial phase hyperenhancement (APHE) followed by late (>60 seconds after injecting UCA) washout of mild degree to diagnose arterially hyper-enhancing HCC using CEUS [18,19].
The Liver Imaging Reporting and Data System recommends CEUS as the initial diagnostic modality and suggests diagnostic criteria to reduce the examiner-dependent bias; i.e., HCC can be definitely diagnosed if observations ≥1 cm show APHE followed by late (>60 seconds) and mild washout (CEUS LR-5). Observations that show rim APHE, early (<60 seconds) washout or marked washout, indicating probably or definitely malignant observations but not HCC specifically, are assigned the CEUS LR-M category (Table 2) [21].
CONCLUSIONS
For the differential diagnosis of focal hepatic lesions, CEUS is comparable to dynamic liver CT. Moreover, CEUS has been used to facilitate the determination of therapeutic targets in loco-regional treatment of HCC and is very useful for evaluation during or after treatment. It is also expected to be useful in evaluating the response to target therapies such as sorafenib because UCA exists only within the vessel. Furthermore, CEUS has been used for the pre-examination of blood vessel condition of donors and recipients before liver transplantation. In conclusion, the use of CEUS in clinical practice is expected to continue to expand, and further studies to validate its usefulness are needed.
Notes
The authors have no conflicts to disclose.