Articles
- Page Path
- HOME > J Liver Cancer > Volume 22(2); 2022 > Article
-
Case Report
Novel management of expected post-radiotherapy complications in hepatocellular carcinoma patients: a case report -
Sung Hoon Chang1*
 , Tae Suk Kim1*
, Tae Suk Kim1* , Yong Hwan Jeon2
, Yong Hwan Jeon2 , Nuri Hyun Jung3
, Nuri Hyun Jung3 , Dae Hee Choi1
, Dae Hee Choi1
-
Journal of Liver Cancer 2022;22(2):183-187.
DOI: https://doi.org/10.17998/jlc.2022.08.03
Published online: August 24, 2022
1Department of Internal Medicine, Kangwon National University Hospital, Kangwon National University School of Medicine, Chuncheon, Korea
2Department of Radiology, Kangwon National University Hospital, Kangwon National University School of Medicine, Chuncheon, Korea
3Department of Radiation Oncology, Kangwon National University Hospital, Kangwon National University School of Medicine, Chuncheon, Korea
-
Corresponding author: Dae Hee Choi Department of Internal Medicine, Kangwon National University Hospital, Kangwon National University School of Medicine, 1 Kangwondaehak-gil, Chuncheon 24341, Korea
Tel. +82-33-258-9058, Fax. +82-33-258-2146 E-mail: dhchoi-md@kangwon.ac.kr - *These two authors contributed equally to this work.
Copyright © 2022 The Korean Liver Cancer Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,630 Views
- 51 Downloads
Abstract
- In recent years, radiotherapy (RT) has been used to treat hepatocellular carcinoma (HCC) at each stage. This clinical trend has developed with the increasing improvement of RT techniques, which show clinical results comparable to those of other treatment modalities. Intensity-modulated radiotherapy uses a high radiation dose to improve treatment effectiveness. However, the associated radiation toxicity can damage adjacent organs. Radiation-induced gastric damage with gastric ulcers is a complication of RT. This report presents a novel management strategy for preventing post-RT gastric ulcers. We present the case of a 53-year-old male patient diagnosed with HCC, who experienced gastric ulcer after RT. Before the second round of RT, the patient was administered a gas-foaming agent, which was effective in preventing RT complications.
- Hepatocellular carcinoma (HCC) is one of the most common primary cancers, and is the sixth most common cancer both worldwide and in South Korea.1,2 Several treatment options are available for managing patients with HCC, but curative modalities are limited as most cases are diagnosed at an intermediate or advanced stage.3 Although many treatment options exist for HCC patients, and each treatment modality has advanced in the past decades, the 5-year overall survival rate continues to be less than 20%.2 In the past, radiotherapy (RT) has been used as a salvage or palliative treatment, and only a few guidelines refer to the role of RT. The use of RT is complicated as it is difficult to avoid radiation exposure to normal hepatocytes and adjacent organs. In the modern era, RT has emerged as a treatment for HCC.4 As RT techniques have improved, intensity-modulated radiotherapy (IMRT), stereotactic ablative body radiotherapy, and charged particle therapy have been used to treat HCC. However, radiation-induced gastric damage (RIGD) commonly occurs as a side effect of upper abdominal RT.5 Most cases of RIGD are mild and self-limiting; however, late toxicities, including ulcerative bleeding, perforation, and stenosis, are potentially life threatening. Here, we report the case of a 53-year-old male patient with HCC who was treated with a gas foaming agent before RT to prevent RIGD due to radiation toxicity. This case report is described in accordance with the CARE guidelines (available at https://www.care-statement.org/).
INTRODUCTION
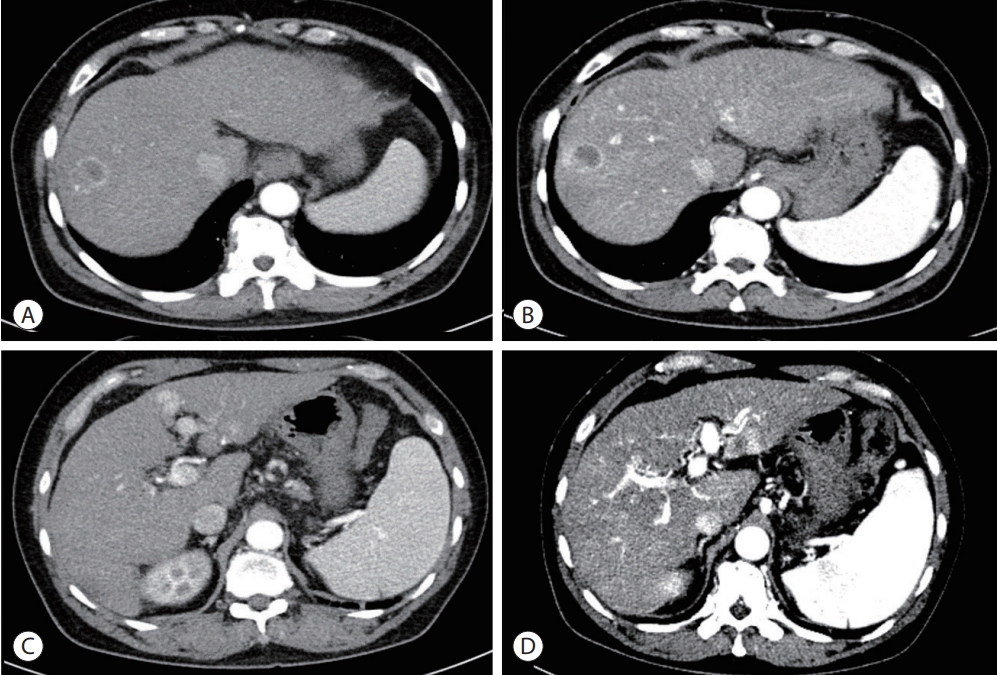
- A 53-year-old male with a history of alcoholic liver cirrhosis was transferred for computed tomography (CT) to further evaluate suspected HCCs in segment 3 and segment 8 of the liver (Fig. 1). On the CT image, the S8 mass showed a rim enhancement pattern and central hypo-enhancement in the arterial phase, and insufficient washout in the portal/delayed phase. The subcapsular portion of the S3 liver segment showed insufficient enhancement to diagnose HCC. Magnetic resonance imaging to diagnose HCC was unsuccessful due to lack of patient cooperation. In the initial laboratory tests, the alpha-fetoprotein level was 5.7 ng/mL and the protein level induced by vitamin K absence II was 41.04 mAU/ mL. A platelet count of 147,000/µL, prothrombin time of 11.9 sec, albumin level of 3.8 g/dL, total bilirubin level of 0.8 mg/dL, alanine aminotransferase level of 27 IU/L and aspartate aminotransferase level of 37 IU/L were obtained. The patient had no history of hepatic encephalopathy or ascites. Therefore, his liver function was considered good, with a Child-Pugh score of 5 (Class A). The performance status was also good, with an Eastern Cooperative Oncology Group status of 0. The patient had Modified Union for International Cancer Control (UICC) stage II, T2N0M0, and the Barcelona Clinic Liver Cancer (BCLC) stage was BCLC A. Based on the modified UICC and BCLC staging systems, embolization, ablation, and liver transplantation were considered as treatments for HCC. After a multidisciplinary approach, transarterial chemoembolization (TACE) was performed for diagnostic or therapeutic purposes, considering patient compliance, socioeconomic status, and reluctance to undergo surgery.
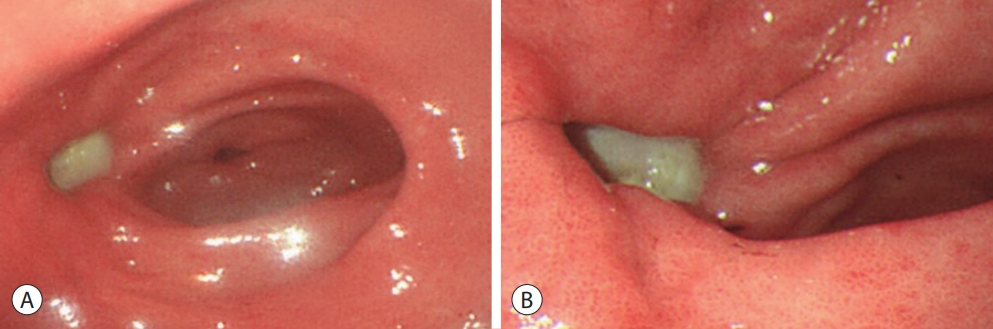
- Angiography revealed hypervascular tumor staining, and chemoembolization was performed for the S3 and S8 lesions. A follow-up CT scan after 1 month showed insufficient lipiodol uptake in the S3 lesion. RT of the S3 lesion comprising 10 fractions totaling 62 Gy was performed. However, 1 month later, the patient complained of dyspepsia and epigastric pain. Esophagogastroduodenoscopy revealed an acute gastric ulcer in the anterior wall of the antrum (Fig. 2). The patient was given ulcer medication and his symptoms improved.
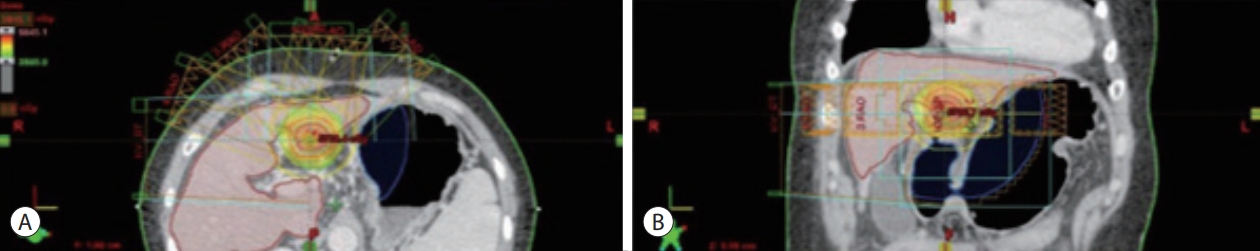
- Nine months later, the size of the enhanced lesion (S3) increased slightly because the radiation dose was insufficient for the tumor location: the lesion was close to the edge of the stomach. Since it was not possible to attempt high-dose RT due to the risk of gastric ulcer, TACE was considered an alternative method. However, this approach failed when the patient was unable to cooperate. Although RT was considered as an option, the risk of complications such as gastric ulcers was a limitation. Radiation directly to the tumor in S3, as was performed in the previous RT round, could worsen the gastric ulcer. To this end, sodium bicarbonate/tartaric acid (a gas-foaming agent) was used in order to prevent a radiation-induced gastric ulcer. A second RT of 46 Gy/23 fx was performed for the S3 lesion. For each RT, 4.0 g of the gas-foaming agent was used for the patient who weighed 65 kg. In addition, scopolamine was applied to suppress intestinal movement 1 hours before treatment. We also performed cone-beam CT before and after all radiation treatments, and an insignificant change was confirmed compared to the planned target volume (Fig. 3). The patient’s prior symptoms of dyspepsia and epigastric pain did not recur after RT with the gas-foaming agent. A recent CT scan showed that the S3 lesion had decreased markedly, without any viable portion (Fig. 4).
CASE REPORT
- Here we report a case in which we introduced an alternative method to prevent complications during RT for HCC. After multidisciplinary consultation, TACE was performed as the second treatment option. As TACE failed, with no uptake into the tumor lesion, RT was performed next. The S3 lesion did not improve on follow-up CT; therefore, additional RT was considered. To address gastric ulcer complications associated with RT, a gas-foaming agent was used.
- Considering the BCLC/TNM stage, surgery was recommended; however, the second option (TACE) was chosen because of the patient’s socioeconomic status and preferences. In a study by Kim et al.,6 more than half of patients with early stage HCC underwent TACE as the first-line treatment. In some cases, the treatment modalities chosen in actual clinical practice differed from the BCLC treatment guidelines.
- A high dose of radiation can be used in IMRT, sparing normal tissue even when the tumor is located near critical organs.7 Broadly speaking, the irradiation tolerance of the stomach is intermediate between that of the small intestine and rectum. Local complications, such as gastric ulcers, can occur as a consequence of RT. Radiation doses to the entire organ between 45 Gy and 50 Gy rarely cause significant RIGD. According to Lee et al.,8 the incidence rate of gastroduodenal ulcers after IMRT is 9.1%. Overall, severe late effects have been estimated to occur in roughly 0.5-3.0% of patients receiving radical RT of the gastrointestinal tract.9 Recommended preventive strategies include proton pump inhibitors, meticulous irradiation techniques, oversight of fractionation, and control of stomach volume.
- In this case, a gastric ulcer had already developed after RT, and therefore, it was necessary to find a prophylactic measure before the next RT in order to prevent another gastric ulcer. Despite having never tried this technique before, based on the recommendation of the radiologist, treatment based on the lateral electron disequilibrium was adopted. Practically, an air-filled stomach induces movement of the stomach position (the gastric ulcer site), which reduces the radiation dose and prevents complications. Theoretically, an alteration in the radiation dose due to electron scattering in tissues with different densities (lateral electron disequilibrium) can reduce the radiation dose.10,11 By applying this principle to this case, we increased the distance between the HCC and the stomach, and decreased the radiation effect. This trial could not be repeated because the amount of air was not constant, and the stomach wall was not positioned as expected. Practically, we presumed that complications would be reduced owing to the following mechanisms: anatomical changes in the stomach after air movement, origin of the radiation area, and the distal antrum anterior wall side being located outside the radiation field. However, this treatment is limited as it is difficult to predict the side effects of stomach filling agents, and further efforts are needed to prevent the occurrence of RTinduced gastric ulcers.
DISCUSSION
-
Conflicts of Interest
The authors have no conflicts of interest to disclose.
-
Ethics Statement
The Institutional Review Board (IRB) of the Kangwon National University Hospital waived the requirement for ethics approval and informed consent (IRB No. KNUH-2022-06-009).
-
Funding Statement
This study was supported by the Research Grant from Institute of Medical Sciences, Kangwon National University 2021.
-
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed for this case report.
-
Author Contribution
Conceptualization: DHC
Data curation: SHC, YHJ NHJ
Formal analysis: SHC, TSK, YHJ, NHJ, DHC
Project administration: TSK, DHC
Resources: SHC, TSK, YHJ, NHJ
Writing original draft: SHC, TSK
Writing review & editing: TSK, NHJ, DHC
Approval of final manuscript: all authors
Article information

- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209−249.ArticlePubMedPDF
- 2. Hong S, Won YJ, Lee JJ, Jung KW, Kong HJ, Im JS, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat 2021;53:301−315.ArticlePubMedPMCPDF
- 3. Delis SG, Dervenis C. Selection criteria for liver resection in patients with hepatocellular carcinoma and chronic liver disease. World J Gastroenterol 2008;14:3452−3460.ArticlePubMedPMC
- 4. Villanueva A. Hepatocellular carcinoma. N Engl J Med 2019;380:1450−1462.ArticlePubMed
- 5. McKay MJ, Foster R. Pathobiology, irradiation dosimetric parameters and therapy of radiation-induced gastric damage: a narrative review. J Gastrointest Oncol 2021;12:3115−3122.ArticlePubMedPMC
- 6. Kim BK, Kim SU, Park JY, Kim DY, Ahn SH, Park MS, et al. Applicability of BCLC stage for prognostic stratification in comparison with other staging systems: single centre experience from longterm clinical outcomes of 1717 treatment-naïve patients with hepatocellular carcinoma. Liver Int 2012;32:1120−1127.ArticlePubMed
- 7. Hwang SY, Lee SM, Im JW, Kim JS, Ahn SB, Ji EK, et al. A case of perforation of gastric ulcer after complete remission of huge hepatocellular carcinoma invading main portal vein with combination therapy of stereotactic body radiation therapy and sorafenib. J Liver Cancer 2014;14:46−52.Article
- 8. Lee KJ, Yoon HI, Chung MJ, Park JY, Bang S, Park SW, et al. A comparison of gastrointestinal toxicities between intensity-modulated radiotherapy and three-dimensional conformal radiotherapy for pancreatic cancer. Gut Liver 2016;10:303−309.ArticlePubMedPMC
- 9. Andreyev J. Gastrointestinal symptoms after pelvic radiotherapy: a new understanding to improve management of symptomatic patients. Lancet Oncol 2007;8:1007−1017.ArticlePubMed
- 10. Disher B, Hajdok G, Gaede S, Battista JJ. An in-depth Monte Carlo study of lateral electron disequilibrium for small fields in ultra-low density lung: implications for modern radiation therapy. Phys Med Biol 2012;57:1543−1559.ArticlePubMed
- 11. Hannallah D, Zhu TC, Bjärngard BE. Electron disequilibrium in high-energy x-ray beams. Med Phys 1996;23:1867−1871.ArticlePubMedPDF
References
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation
- Download Citation
- Close
- Related articles
-
- Multidisciplinary approach for hepatocellular carcinoma patients: current evidence and future perspectives
- Current perspectives on radiotherapy in hepatocellular carcinoma management: a comprehensive review
- Management of early-stage hepatocellular carcinoma: challenges and strategies for optimal outcomes
- Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab
- Stereotactic body radiation therapy for elderly patients with small hepatocellular carcinoma: a retrospective observational study

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION


 Follow JLC on Twitter
Follow JLC on Twitter