Systemic therapy for advanced hepatocellular carcinoma: consideration for selecting second-line treatment
Article information
Abstract
Several molecular-targeted agents have been tested as first- or second-line therapies for hepatocellular carcinoma (HCC) but failed to improve clinical outcomes; sorafenib has been the only approved systemic agent for treating HCC for almost 10 years. Regorafenib resulted in a significant improvement in overall survival and thus was approved for HCC patients previously treated with sorafenib. Subsequently, cabozantinib and ramucirumab demonstrated superior overall survival compared with placebos in phase III clinical trials. Immune checkpoint inhibitors such as nivolumab with or without ipilimumab and pembrolizumab are also available in some countries for patients who are unresponsive to sorafenib. Some second-line agents are available for patients who are unresponsive to sorafenib; however, little is known about the considerations for selecting appropriate second-line systemic agents. Hence, this study aimed to review the current and future perspectives of second-line systemic agents.
INTRODUCTION
Systemic therapy for hepatocellular carcinoma (HCC) has changed markedly since 2007 following the approval of sorafenib. Various molecular-targeted agents have subsequently been tested as first- or second-line therapies but have failed to improve clinical outcomes, and sorafenib has remained the only approved systemic agent for HCC for almost 10 years. Regorafenib significantly improved overall survival (OS) and was approved for HCC patients previously treated with sorafenib in 2017. Cabozantinib and ramucirumab demonstrated superior OS compared with placebos in phase III clinical trials. Immune checkpoint inhibitors such as nivolumab with or without ipilimumab and pembrolizumab had relatively high response rates and durable response in phase I/II clinical trials; they are also available as treatment for patients who are unresponsive to sorafenib in some countries. A couple of second-line agents can be used to treat patients who had progression during or are intolerant to sorafenib treatment; however, little is known about the different considerations for selecting a second-line systemic agent and methods for optimizing the sequencing of therapy. Hence, this study aimed to review the current and future perspectives of second-line systemic agents.
SECOND-LINE SYSTEMIC AGENTS
1. Molecular-targeted agents
1) Regorafenib
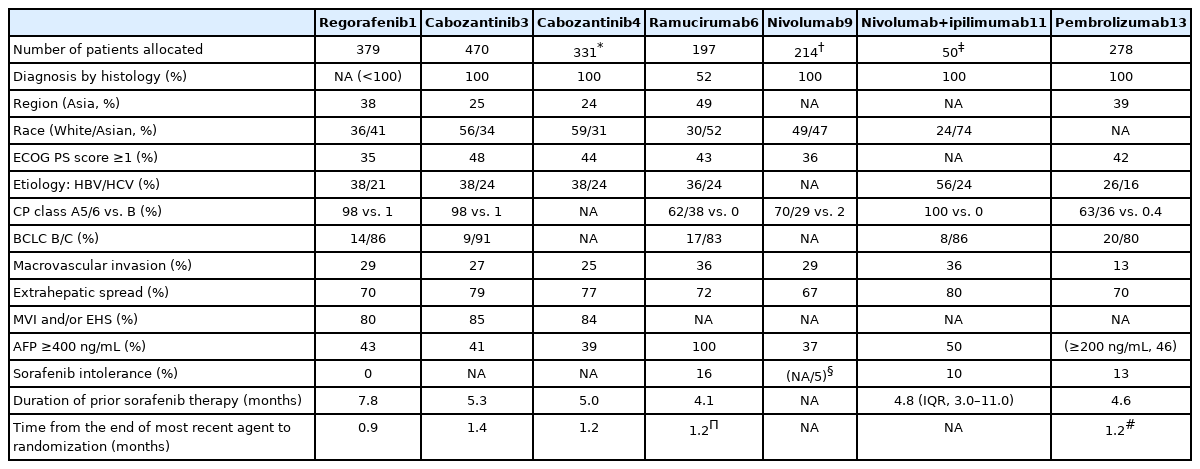
Regorafenib inhibits multiple protein kinases, including the vascular endothelial growth factor receptors (VEGFRs) 1–3 and Tie. A randomized, double-blind, placebo-controlled phase 3 trial (RESORCE) enrolled 573 patients who tolerated sorafenib well (≥400 mg/day for ≥20 days of the last 28 days of treatment) and whose disease progressed during sorafenib treatment (Table 1).1 Patients who did not tolerate sorafenib were excluded since regorafenib and sorafenib have similar toxicity profiles (Table 2). Patients were randomized in a 2:1 ratio to receive regorafenib 160 mg or placebo once daily at weeks 1–3 of each 4-week cycle.
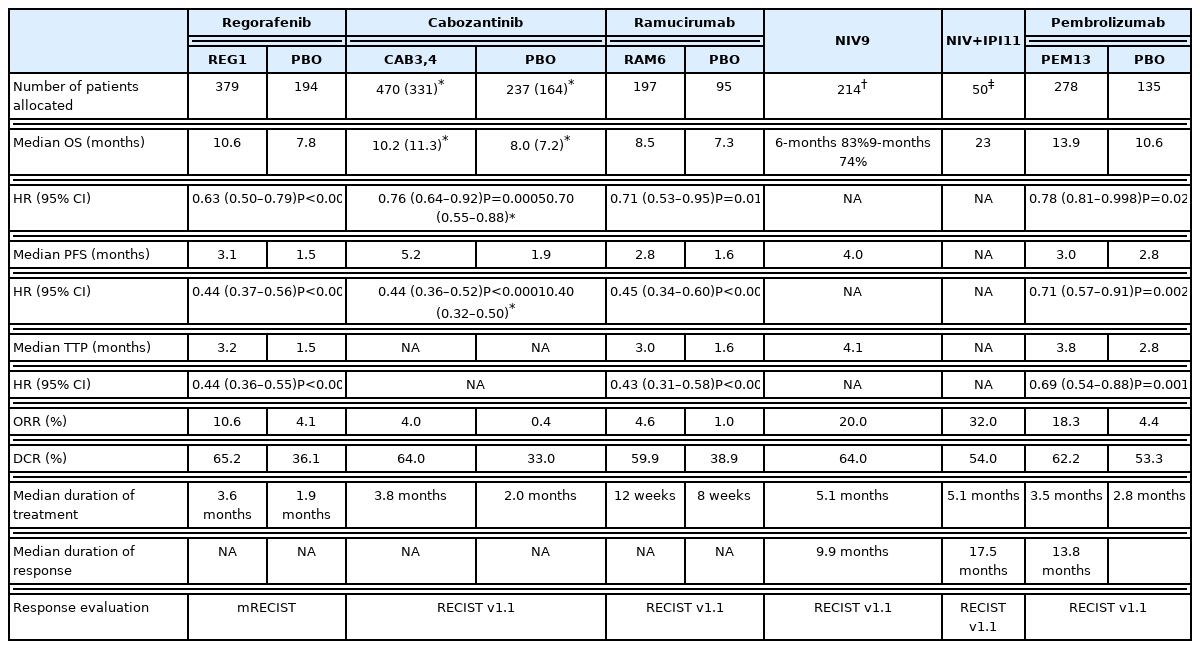
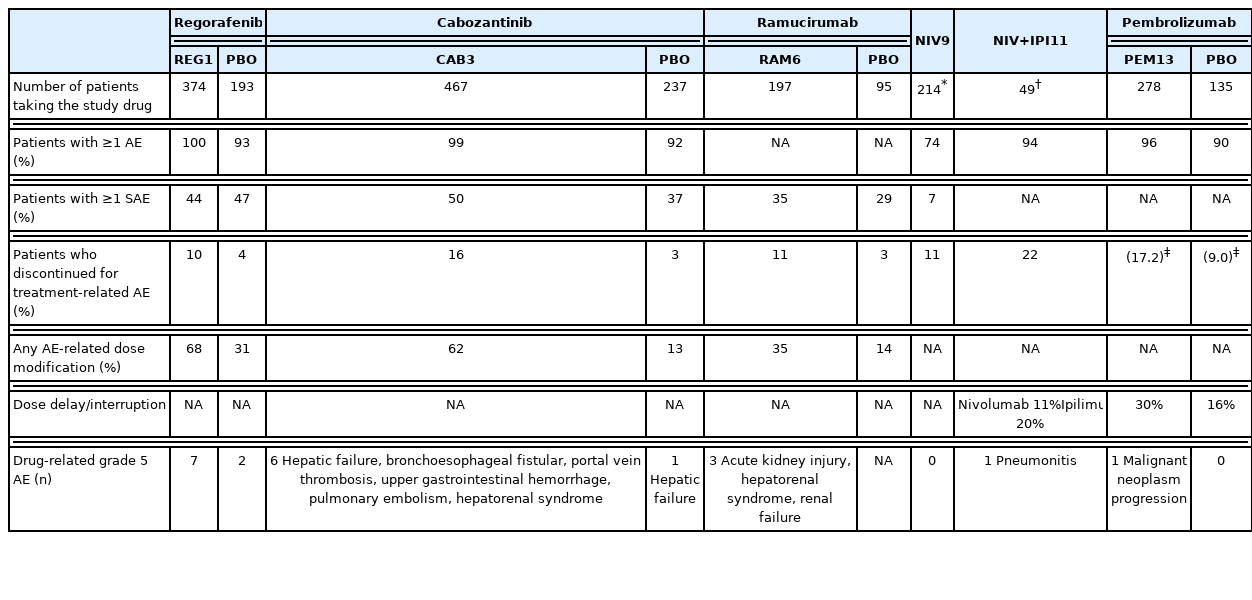
Regorafenib improved the OS with a hazard ratio (HR) of 0.63 (median, 10.6 vs. 7.8 months; 95% confidence interval [CI], 0.50–0.79; one-sided P <0.001; Table 3).1 The median progression-free survival (PFS) durations determined based on the modified Response Evaluation Criteria in Solid Tumors were 3.1 months for patients treated with regorafenib and 1.5 months for those treated with placebo (HR, 0.46; 95% CI, 0.37–0.56; one-sided P <0.001). The disease control rate was 65% (complete response, 1%; partial response, 10%).1 The occurrence of regorafenib-related adverse events resulted in interruptions or dose reductions in 68% of patients (vs. 10% in the placebo group) and discontinuation in 10% of patients (vs. 4% in the placebo group) (Table 4). The most common clinically relevant grade 3 or 4 adverse events were hypertension (15%), hand-foot skin reaction (13%), fatigue (9%), and diarrhea (3%), as shown in Table 5.1
Based on the data from the RESORCE trial, regorafenib was approved for advanced HCC as a second-line therapy. However, whether patients who were intolerant to sorafenib can tolerate and respond to regorafenib well must still be elucidated, since those patients were excluded from the RESORCE trial.
A post-hoc analysis found that longer time-to-progression during sorafenib treatment corresponds to longer time-to-progression during regorafenib treatment (median: 2.8, 3.9, 4.2, and 4.5 months for patients with the first, second, third, and fourth quartiles of time-to-progression on sorafenib, respectively). The time-to-progression benefit was consistent with HRs ranging from 0.26 to 0.66.2
2) Cabozantinib
Cabozantinib is an oral, multi-tyrosine kinase inhibitor that targets VEGFR, mesenchymal-epithelial transition (MET), growth arrest-specific 6 gene receptor (Axl), RET, KIT, and FMS-like tyrosine kinase 3. Cabozantinib has a potent inhibitory effect on VEGFR and MET. A randomized, double-blind, placebo-controlled phase III trial (CELESTIAL) enrolled 707 advanced HCC patients who received up to two lines of previous systemic therapy (including sorafenib) and whose disease progressed after receiving one of these treatments.3 Patients were randomized in a 2:1 ratio to receive 60 mg cabozantinib or placebo once daily.
The median OS was significantly longer in the cabozantinib-treated group (10.2 months for cabozantinib vs. 8.0 months for placebo; HR, 0.76; 95% CI, 0.63–0.92; P =0.005). The median PFS durations were 5.2 and 1.9 months in the cabozantinib- and placebo-treated groups, respectively (HR, 0.44; 95% CI, 0.36–0.52; P <0.001). The objective response rate was 4% (vs. 0.4% for placebo), and the disease control rate was 64% (vs. 33% for placebo). The occurrence of treatment-related adverse events led to therapy discontinuation in 16% of the cabozantinib group (vs. 3% in the placebo group). The most common grade 3 or 4 adverse events were hand-foot skin reaction (17%), hypertension (16%), and increased aspartate aminotransferase levels (12%). The most common adverse events of any grade leading to dose reductions in the cabozantinib-treated group were hand-foot skin reaction (22%), diarrhea (10%), fatigue (7%), hypertension (7%), and increased aspartate aminotransferase levels (6%).
A subgroup analysis of patients previously treated with sorafenib alone (331 in the cabozantinib-treated group and 164 in the placebo-treated group) also revealed that cabozantinib improved the OS (median, 11.3 vs. 7.2 months; HR, 0.70; 95% CI, 0.55–0.88) and PFS (median, 5.5 vs. 1.9 months; HR, 0.40; 95% CI, 0.32–0.50).4 The objective response rate was 5%. The median OS durations were 8.9, 11.5, and 12.3 months for patients administered sorafenib for <3, 3 to <6, and ≥6 months, respectively. The median PFS also tended to increase as the durations of previous sorafenib therapy increased (3.8, 5.4, and 5.7 months for those who received sorafenib for <3, 3 to <6, and ≥6 months, respectively).4 The survival benefit was observed irrespective of the duration of previous sorafenib therapy with HRs ranging from 0.65 to 0.82 for OS and from 0.35 to 0.48 for PFS.4
3) Ramucirumab
Ramucirumab is a recombinant IgG1 monoclonal antibody that binds to VEGFR-2. A previous randomized, placebo-controlled, phase III trial (REACH) that enrolled HCC patients previously treated with sorafenib failed to show superiority of ramucirumab over placebo in terms of OS (9.2 months vs. 7.6 months; HR, 0.87; 95% CI, 0.72–1.05; P =0.14).5 However, a previous subgroup analysis revealed an improvement in OS among patients with a baseline alpha-fetoprotein (AFP) level of ≥400 ng/mL who were administered ramucirumab (7.8 vs. 4.2 months; HR, 0.67; 95% CI, 0.51–0.90). Subsequently, the REACH-2 study on the use of ramucirumab in advanced HCC patients with baseline AFP levels of 400 ng/mL or more enrolled 292 patients and randomized them in a 2:1 ratio to receive 8 mg/kg intravenous ramucirumab or a placebo every 2 weeks.
Ramucirumab significantly improved the OS compared with placebo (8.5 months vs. 7.3 months; HR, 0.71; 95% CI, 0.53–0.95; P =0.02).6 The objective response and disease control rates were 4.6% (vs. 1.1%; P =0.17) and 59.9% (vs. 38.9%; P =0.0006), respectively. The occurrence of treatment-related adverse events led to therapy discontinuation in 11% of the ramucirumab-treated group and in 3% of the placebo-treated group. The most common grade 3 or worse treatment-emergent adverse events were hypertension (13%), hyponatremia (6%), and increased aspartate aminotransferase levels (3%). Bleeding events (24% vs. 13%), liver injury or failure (40% vs. 30%), and infusion-related reactions (9% vs. 3%) were observed more frequently in the ramucirumab-treated group.6
The median OS of the patients treated with ramucirumab in the REACH-2 trial was relatively shorter than that of patients treated with other second-line agents; however, the elevated AFP level of enrolled patients might be a plausible explanation for this, since a high AFP level is associated with poor prognosis.7
2. Immunotherapy
Immune checkpoints are co-inhibitory proteins that prevent T cells from attacking other cells in the body. Immune checkpoint inhibitors can negatively impact the immune system and restore immune responses against cancer cells. The targets of immune checkpoint inhibitors include cytotoxic T-lymphocyte protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed cell death ligand 1.8 Most immune checkpoint inhibitors have exhibited relatively high tumor response rates and response durability in phase I/II trials; therefore, they have become available in some countries such as the United States and South Korea. However, successful phase III trials have not yet been reported since confirmatory phase III trials either failed to meet their primary endpoints or are currently ongoing.
1) Nivolumab
Nivolumab is a fully humanized IgG4 monoclonal antibody against human PD-1. A phase I/II, open-label dose escalation and expansion trial of nivolumab monotherapy (CHECKMATE-040) was conducted in advanced HCC patients and included those whose disease progressed after treatment with at least one systemic therapy or who were intolerant to or refused sorafenib treatment.9 In the dose-expansion phase, 214 advanced HCC patients received intravenous nivolumab (3 mg/kg every 2 weeks).9 The objective response rate and disease control rate were 20% and 64%, respectively (3 complete responses, 39 partial responses, and 96 stable diseases).9 The median time to progression was 4.1 months in the dose-expansion phase. The 9-month OS rate was 74%.9 Grade 3 or 4 treatment-related adverse events were observed in 40 of 214 patients (19%) in the dose-expansion phase (increased aspartate aminotransferase levels occurred in 4% of the patients and increased alanine aminotransferase levels in 2% of the patients).9
The CHECKMATE-040 study enabled the accelerated approval of nivolumab by the US FDA for treating HCC in patients who had been previously treated with sorafenib in 2017. However, a confirmatory randomized phase III trial of nivolumab versus sorafenib as first-line treatment (CHECKMATE-459, NCT02576509) was unable to achieve improved OS as a primary endpoint (16.4 months in the nivolumab group vs. 14.7 months in the sorafenib group; HR, 0.85; 95% CI, 0.72–1.02; P =0.0752).10
2) Nivolumab plus ipilimumab
Ipilimumab is a fully humanized IgG1 monoclonal anti-CTLA4 antibody. A phase I/II, open-label, multi-cohort study investigating nivolumab and nivolumab-based combinations in advanced HCC patients (CHECKMATE-040) also evaluated the safety and efficacy of nivolumab plus ipilimumab in three different dosing regimens.11 Patients were randomized in a 1:1:1 ratio to either receive 1 mg/kg of nivolumab plus 3 mg/kg of ipilimumab, administered every 3 weeks (4 doses), followed by 240 mg of nivolumab every 2 weeks (n=50); 3 mg/kg of nivolumab plus 1 mg/kg of ipilimumab, administered every 3 weeks (4 doses), followed by 240 mg of nivolumab every 2 weeks (n=49); or 3 mg/kg of nivolumab every 2 weeks plus 1 mg/kg of ipilimumab every 6 weeks (n=49).11
Patients who received nivolumab 1 mg/kg plus ipilimumab 3 mg/kg had better outcomes, with an objective response rate of 32% (complete response, 4; partial response, 12) and a median OS of 22.8 months. The disease control rate was 54%, and the median response duration was 17.5 months. Grade 3 or 4 treatment-related adverse events occurred in 26 of 49 patients (53%) treated with 1 mg/kg of nivolumab plus 3 mg/kg of ipilimumab (increased aspartate aminotransferase levels, 16%; increased lipase levels, 12%; and increased alanine aminotransferase levels, 12%). The incidence of adverse events among patients who received nivolumab plus ipilimumab therapy was higher than those who underwent nivolumab monotherapy. Immune-mediated adverse events were also more frequently reported (Table 5). The CHECKMATE-9DW study on nivolumab combined with ipilimumab versus sorafenib or lenvatinib in advanced HCC patients who had not received prior systemic therapy is currently ongoing (NCT04039607).
3) Pembrolizumab
Pembrolizumab is another humanized IgG4 monoclonal antibody that targets PD-1. A phase II study of pembrolizumab (KEYNOTE-224) reported an objective response rate of 16% and a disease control rate of 62% in advanced HCC patients previously treated with sorafenib.12 However, a randomized placebo-controlled phase III study of pembrolizumab in advanced HCC patients as a second-line therapy (KEYNOTE-240) did not achieve improved OS and PFS as co-primary endpoints.13 The median OS durations were 13.9 months and 10.6 months in the pembrolizumab-treated and placebo-treated groups, respectively (HR, 0.78; 95% CI, 0.61–0.99; P =0.0238). The corresponding median PFS durations were 3.0 months and 2.8 months, respectively (HR, 0.72; 95% CI, 0.57–0.90; P =0.0022). Pembrolizumab improved OS and PFS compared with the placebo; however, the predetermined statistical significance level was not reached. The objective response and disease control rates were 18.3% (vs. 4.4% for placebo) and 62.2% (vs. 53.3% for placebo), respectively. The most common grade 3 or 4 adverse events were increased aspartate aminotransferase (13.3%), bilirubin levels (7.5%), and alanine aminotransferase levels (6.1%). Although pembrolizumab is also approved as treatment for any type of solid tumor with high microsatellite instability or deficient mismatch repair, the incidence of high microsatellite instability is reportedly <3% in HCC patients.14
A recent subgroup analysis of the KEYNOTE-240 trial revealed a trend toward greater benefit of pembrolizumab treatment in the Asian subgroup than in the overall cohort.15 The median OS was 13.8 months (vs. 8.3 months for placebo) (HR, 0.55; 95% CI, 0.37–0.80), while the median PFS was 2.8 months (vs. 1.4 months for placebo) (HR, 0.48; 95% CI, 0.32–0.70).15 The objective response and disease control rates were 20.6% (vs. 2.0% for placebo) and 59.8% (vs. 40.0% for placebo), respectively, and the safety profiles were comparable. 15 The regional differences or underlying epidemiology might contribute to this differential benefit; however, further validation studies are warranted to confirm this finding.
3. Considerations for selecting second-line systemic agents
As described above, a couple of second-line systemic agents are available if HCC patients are unresponsive to sorafenib treatment. These agents have significantly improved OS or showed a good response; however, little is known about the different considerations when selecting an agent and optimizing the sequencing of therapy.
1) Previous sorafenib treatment
When choosing a systemic agent for advanced HCC, clinicians should consider the efficacy, safety, predictive factors of response, and so on. As regards choosing the appropriate second-line therapy for advanced HCC, tolerability to sorafenib is one of the first key guidelines. The RESORCE trial enrolled patients whose disease progressed after receiving sorafenib and who were treated with sorafenib at a dose of ≥400 mg/day for 20 days in the last 28 days only. One of the REACH-2 trial inclusion criteria was patients who received sorafenib for ≥14 days. If patients are intolerant to sorafenib, it is very unlikely that they will tolerate regorafenib because the two agents share similar chemical structures and toxicity profiles.
The time to progression in patients previously treated with sorafenib can also be a good indicator for selecting the appropriate second-line agents. Regorafenib showed a benefit compared with placebo in terms of the time to progression after treatment with sorafenib; however, a longer time to progression in patients treated with sorafenib confers a longer time to progression in those treated with regorafenib.2 Although cabozantinib also showed better PFS than placebo irrespective of the duration of previous sorafenib therapy, the PFS was shorter in patients who previously received sorafenib therapy for <3 months compared with those who received the treatment for a longer duration.4 These results may imply that patients previously treated with sorafenib who showed a longer time to progression can also benefit from another tyrosine kinase inhibitors.
A recent matching-adjusted indirect comparison analysis using the real-world data of patients treated with regorafenib and subgroup data of patients from the CELESTIAL trial who received cabozantinib as a second-line therapy also reported that the median OS in both groups increased as the duration of sorafenib therapy increased.16 In the regorafenib-treated group, the median OS durations were 6.5 months (vs. 9.5 months for cabozantinib; HR, 0.68; 95% CI, 0.39–1.16), 8.0 months (vs. 11.5 months for cabozantinib; HR, 0.66; 95% CI, 0.42–1.01), and 13.4 months (vs. 12.3 months for cabozantinib; HR, 0.89; 95% CI, 0.52–1.51) in patients who received sorafenib for <3 months, 3 to <6 months, and ≥6 months, respectively.16 Although the differences between real-world data and well-monitored clinical trial data might contribute to the differences in OS between regorafenib and cabozantinib, patients whose disease progressed after treatment with sorafenib for <6 months tended to have improved survival benefits from cabozantinib therapy.16
2) Alpha-fetoprotein
Another evident finding is the serum AFP level. The REACH-2 trial included patients with serum AFP levels of ≥400 ng/mL; therefore, ramucirumab cannot be administered to those with a serum AFP of <400 ng/mL.
The CELESTIAL trial enrolled patients regardless of their serum AFP level; however, the median OS was slightly longer in patients with low baseline AFP levels as expected based on the cutoff level of 400 ng/mL.17 The median OS durations were 13.9 months and 10.3 months in the cabozantinib-treated and placebo-treated groups (HR, 0.81; 95% CI, 0.62–1.04) for patients with a baseline AFP level of <400 ng/mL, 8.5 months in the cabozantinib-treated group and 5.2 months in the placebo-treated group (HR, 0.71; 95% CI, 0.54–0.94) for patients with baseline AFP levels of ≥400 ng/mL.17 Additionally, the median OS durations were 16.1 months in patients with an AFP response (defined as ≥20% decrease from the baseline value) and 9.1 months in those without an AFP response (HR, 0.61; 95% CI, 0.45–0.84) in the cabozantinib-treated group.17 In patients treated with ramucirumab, the median OS durations in patients with AFP response was significantly longer than those observed in those without AFP response (13.6 vs. 5.6 months; HR, 0.45; 95% CI, 0.35–0.57; P <0.0001). Although the AFP response was an independent predictor for OS,17 it can hardly be useful when choosing the appropriate second-line agents because of the nature of “on-treatment” predictors.
3) Efficacy
No study performing head-to-head prospective comparison of second-line agents for advanced HCC has been conducted to date. In the absence of head-to-head clinical trial data, indirect comparisons, such as network meta-analysis or matching-adjusted indirect comparison method, may be helpful.
In a recent network meta-analysis, regorafenib showed a significant improvement in OS (HR, 0.71; 95% CI, 0.54–0.93) compared with ramucirumab, while no significant difference was observed between regorafenib and cabozantinib (HR, 0.82; 95% CI, 0.62–1.07).18 Regorafenib (HR, 0.74; 95% CI, 0.56–0.98) and cabozantinib (HR, 0.71;95% CI, 0.55–0.92) exhibited improved PFS compared with ramucirumab. However, a previous subgroup analysis of patients with a serum AFP level of ≥400 ng/mL revealed no significant differences among the three agents (regorafenib, cabozantinib, and ramucirumab) in terms of OS and PFS.18
Additional analysis with a matching-adjusted indirect comparison method used individual patient data from the CELESTIAL trial and included a subpopulation of patients who received cabozantinib as a second-line therapy.19 Cabozantinib exhibited comparable OS (median 11.4 months vs. 10.6 months; P =0.3474) and longer PFS (median 5.6 months vs. 3.1 months; P =0.0005) to regorafenib.19 Another analysis using a matching-adjusted indirect comparison method also included a subgroup of patients from the CELESTIAL trial with a serum AFP level of ≥400 ng/mL who previously received sorafenib alone and compared the efficacy of cabozantinib with that of ramucirumab.20 Cabozantinib also demonstrated comparable OS (median 10.6 months vs. 8.7 months; P =0.104) and longer PFS (median 5.5 months vs. 2.8 months; P =0.016) to ramucirumab. However, it should be kept in mind that most of these studies were based on “indirect” comparisons and used study-level data instead of real-world data.
Korean real-world data reported median OS values of 6.9 and 5.9 months for regorafenib and nivolumab, respectively (P =0.77) and objective response rates of 5.9% for regorafenib and 16.7% for nivolumab of HCC patients who were unresponsive to sorafenib treatment.21 The regorafenib-treated patients had slightly longer numerical OS compared to the nivolumab-treated patients; however, a previous multivariate analysis found that nivolumab was associated with prolonged OS (adjusted HR, 0.54; 95% CI, 0.30–0.96; P =0.04).21 Other real-world data demonstrated that the median OS durations were 30.9 and 32.6 weeks in regorafenib-treated patients and nivolumab-treated patients, respectively, after they failed to respond to sorafenib (HR, 0.83; 95% CI, 0.64–1.07; P =0.154).22 The objective response rates were 4.0% and 13.3% in regorafenib-treated patients and nivolumab-treated patients, respectively. Interestingly, the propensity score-matched analysis indicated that PFS was comparable between the regorafenib-treated and nivolumab-treated patients across all subgroups, including those who had received sorafenib for <3 or ≥3 months.22
4) Safety profile
Most patients treated with systemic therapy experience one or more adverse events. Of the three molecular-targeted agents (regorafenib, cabozantinib, and ramucirumab), the percentage of patients who experienced serious adverse events or any adverse event-related dose modification was relatively low in the group treated with ramucirumab (Table 4). The incidence of hypertension among the three agents was comparable, while that of peripheral edema was slightly elevated among those treated with ramucirumab. Hand-foot skin reaction or diarrhea developed more frequently in patients treated with regorafenib or cabozantinib, while bleeding events occurred more frequently in those treated with ramucirumab.
Approximately 11–22% of patients receiving immune checkpoint inhibitors experienced adverse events leading to treatment discontinuation (Table 4). The proportion of patients who discontinued treatment due to adverse events was the highest (22%) in the group administered a combination of nivolumab (1 mg/kg) and ipilimumab (3 mg/kg).11 Among 49 patients, three, three, and two patients discontinued the treatment due to pneumonitis, hepatitis, and diarrhea/colitis, respectively.20 All these events were regarded as immune-related adverse events, and one patient died from pneumonitis (grade 5 adverse event).23 Although patients receiving immune checkpoint inhibitors do not develop hand-foot skin reactions, dermatological adverse events such as rash or pruritus occur more frequently.
5) Health-related quality of life
Although OS and PFS are the main outcomes of interest, maintaining quality of life is also important in most advanced cancer patients. A pooled analysis of advanced HCC patients with a serum AFP level of ≥400 ng/mL from REACH and REACH-2 trials was performed to investigate the effects of ramucirumab on patient-reported outcomes.23 The time to deterioration of disease symptoms (measured using the 8-item Functional Assessment of Cancer Therapy Hepatobiliary Symptom Index) was increasingly delayed in ramucirumab-treated patients compared to placebo-treated patients (median 3.3 vs. 1.9 months; HR, 0.73; 95% CI, 0.56–0.94; P =0.0152). The RESORCE trial found no other clinically meaningful differences between the regorafenib-treated and placebo-treated groups.1 Nivolumab therapy did not cause significant changes in patient-reported health status (assessed using the European Quality of Life-5 Dimensions utility index [EQ-5D-3L] and Visual Analogue Scale [EQ-5D-VAS]) from baseline;9 however, the patient-reported health status (measured using the EQ-5D-VAS and EQ-5D-3L) of patients taking nivolumab with ipilimumab improved, although this finding was not obtained from comparative studies.11
6) Neutrophil-to-lymphocyte ratio
The neutrophil-to-lymphocyte ratio (NLR) has been evaluated as a prognostic factor in various types of solid tumors, and has also been studied in HCC patients who underwent surgical resection, transplantation, radiofrequency ablation, transarterial therapy, and sorafenib therapy.24 In HCC patients receiving sorafenib, an NLR of ≥2.3 was an independent indicator of poor OS.24
The median NLR was higher in progressive HCC patients receiving nivolumab compared with those who achieved complete response or partial response;25 moreover, patients with a baseline NLR of ≥3 showed worse OS than those with an NLR of <3.26 Interestingly, a recent study suggested that the incidence of hyperprogressive disease during nivolumab therapy increased as the NLR increased, and patients with a baseline NLR of >6 had a 46% risk of developing hyperprogressive disease.27 High NLR (>4.125) or NLR increase at week 4 could predict the occurrence of hyperprogressive disease. 26,27
4. Limitations
All systemic agents mentioned above have exhibited efficacy and safety in sorafenib-treated patients. However, lenvatinib and atezolizumab plus bevacizumab were approved as first-line treatment for advanced HCC, while all trials on second-line agents have begun and have been conducted for patients treated with sorafenib.
To date, no robust evidence showing patients with disease progression while on or were intolerant to first-line agents other than sorafenib exists. Other treatment options might be carefully offered to patients who remained unresponsive to lenvatinib, similar to those who were unresponsive to sorafenib therapy, since both are tyrosine kinase inhibitors. However, it remains to be elucidated how to sequence treatment after immune checkpoint inhibitor-based therapy (atezolizumab plus bevacizumab) failure. The efficacy of PD-1 inhibitor (nivolumab) after PD-L1 therapy (atezolizumab) failure or that of VEGFR-2 monoclonal antibody (ramucirumab) after VEGFR monoclonal antibody (bevacizumab) is another area of uncertainty.
CONCLUSIONS
Sorafenib has remained the only approved systemic agent for HCC for almost 10 years since 2007. More systemic agents have been used after regorafenib has been approved as second-line therapies; however, limited evidence is available to guide clinicians in choosing the appropriate second-line systemic agents. Hence, clinicians should be aware of each drug’s properties, data on efficacy, safety, tolerability, and patient-reported outcome, and consider which agent might be better and helpful for patients. Patients’ preferences for oral or intravenous administration and their economic burden should be wisely considered. Additional studies to further widen and deepen our understanding for these agents are eagerly anticipated.
Notes
Conflicts of Interest
J-WP has served as a consultant or advisor in Roche, Genetech, BMS, Bayer, Eisai, Ipsen, and AstraZeneca; received honoraria from Bayer and Eisai; and participated in research sponsored by Ono-BMS, AstraZeneca, Blueprint, Roche, Eisai, Exelixis, Kowa, and Merk. BHK has served as an advisor in Eisai and Roche, received honoraria from Abbvie, and participated in research sponsored by Ono-BMS. BHK has no conlicts of interest to disclose.
Ethics Statement
This review article is fully based on articles which have already been published and did not involve additional patient participants. Therefore, IRB approval is not necessary.
Funding Statement
This work was supported by the National Cancer Center (NCC 1810031 and 2020162), Korea.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Author Contribution
Conception and study design: BHK and J-WP
Supervision: J-WP
Data analysis and interpretation: BHK, and J-WP
Writing-original draft: BHK
Writing-review and editing: BHK and J-WP.
All authors reviewed and approved the final draft.