Articles
- Page Path
- HOME > J Liver Cancer > Volume 20(1); 2020 > Article
-
Original Article
A Survey of Liver Cancer Specialists’ Views on the National Liver Cancer Screening Program in Korea -
Won Sohn1*, Young-Sun Lee2*, Jae Geun Lee3, Jihyun An4, Eun Sun Jang5, Dong Ho Lee6, Dong Hyun Sinn7

-
Journal of Liver Cancer 2020;20(1):53-59.
DOI: https://doi.org/10.17998/jlc.20.1.53
Published online: March 31, 2020
1Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
2Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
3Department of Surgery, Yonsei University College of Medicine, Seoul, Korea
4Department of Gastroenterology and Hepatology, Hanyang University College of Medicine, Seoul, Korea
5Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
6Department of Radiology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
7Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
-
Corresponding author : Dong Hyun Sinn Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea
Tel. +82-2-3410-3409, Fax. +82-2-3410-6983 E-mail; dh.sinn@samsung.com - *The first two authors contributed equally to this study.
Copyright © 2020 The Korean Liver Cancer Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 4,286 Views
- 133 Downloads
- 5 Citations
Abstract
-
Background/Aims
- To reduce the cancer burden, the Korean government initiated the National Cancer Control Plan including the National Liver Cancer Screening Program (NLCSP). Ultrasonography examinations and α-fetoprotein tests at six-month intervals are currently offered for high-risk individuals. High-risk individuals are identified by reviewing the National Health Insurance Service claims data for medical use for the past two years using International Classification of Diseases Codes for specific liver disease. We surveyed the attitudes and opinions towards the NLCSP to understand the issues surrounding the NLCSP in Korea.
-
Methods
- Altogether, 90 Korean Liver Cancer Association members participated in online and offline surveys between November and December 2019.
-
Results
- Approximately one-quarter (27%) of the survey participants rated the NLCSP as very contributing and about two-thirds (68%) as contributing to some extent toward reducing hepatocellular carcinoma (HCC)-related deaths in Korea. Most (87.8%) responded that the current process of identifying high-risk individuals needs improvement. Many (78.9%) were concerned that the current process identifies individuals who use medical services and paradoxically misses those who do not. When asked for the foremost priority for improvement, solving ‘duplication issues between the NLCSP and private clinic HCC screening practices’ was the most commonly selected choice (23.3%).
-
Conclusions
- The survey participants positively rated the role of the NLCSP in reducing liver cancer deaths. However, many participants rated the NCLSP as needing improvement in all areas. This survey can be a relevant resource for future health policy decisions regarding the NLCSP in Korea.
- Liver cancer is the sixth most common cancer (fourth in men and sixth in women) and the second-largest cause of cancer mortality in South Korea [1]. A total of 15,771 cases (11,774 men and 3,997 women) were identified, with an agestandardized incidence rate of 18.0 persons per 100,000 (29.2 in men and 7.9 in women) in 2016 [2]. The mortality from liver cancer was 10,721 (7,982 in men and 2,739 in women) in 2017 [2]. To reduce the cancer burden, the Korean government initiated a comprehensive National Cancer Control Plan in 1996 [3]. As part of this plan, the National Cancer Screening Program was launched in 1999 [4,5]. In terms of liver cancer screening, the National Liver Cancer Screening Program (NLCSP) began in 2003 by offering an ultrasonography (US) examination and an α-fetoprotein (AFP) test for people aged 40 years and over who were hepatitis B surface antigen (HBsAg) or anti-hepatitis C virus (HCV)-positive or had liver cirrhosis. The tests were offered at six-month intervals from 2003 to 2011, at one-year intervals from 2012 to 2015, and at six-month intervals since 2016 (https://www.g-health.kr/portal/index.do, accessed at December 21, 2019).
- The stage at diagnosis is an important prognostic factor for cancer patient survival [6]. The five-year relative survival rate is high for localized Surveillance, Epidemiology, and End Results (SEER) stage liver cancer (42.8%) and dismal (2.5%) for distant SEER stage liver cancer [7]. Compared to the US SEER data, Korean patients had better stage distribution and stage-specific survival rates, which the authors suggested might be the result of contributions by the National Cancer Screening Program [7]. However, data on the efficacy of the NLCSP is very limited.
- The Korean Liver Cancer Association (KLCA) is a leading, multidisciplinary society promoting research in liver cancerrelated disciplines, thereby providing a platform for the exchange of knowledge and information and suggesting scientific evidence and guidelines needed to overcome liver cancer with the aim of contributing toward public health. This study conducted a survey of KLCA members to assess their insights and opinions on the NLCSP, understand issues regarding the NLCSP, and provide relevant information for health policy decision-making in Korea.
INTRODUCTION
- 1. Study population and design
- This survey study was conducted by a project committee of the KLCA. The first e-mail requesting participation in the online survey was sent to 735 KLCA members on November 25, 2019. Fifty-three members responded and completed the online survey. The second e-mail requesting participation in the online survey was sent on December 03, 2019, to which 18 members responded and completed the online survey. Lastly, a printed survey was prepared and participation was requested from 70 KLCA members who attended the KLCA single-topic conference held on December 13, 2019. Nineteen members completed the printed survey form. Finally, 90 KLCA members participated in this survey study. The baseline characteristics of the survey participants are summarized in Table 1. This study corresponds to an institutional review board approval waiver as only de-identified survey results were used.
- 2. Survey variables
- The survey comprised three parts. The first part consisted of three questions on sub-specialty, acquisition year of the medical sub-specialty, and place of work. The second part consisted of seven questions asking the KLCA member’s assessment of the status of the NLCSP in Korea. The third part consisted of six questions asking for the KLCA member’s opinion on how the NLCSP could be improved. A complete survey (in the Korean language) can be found in Supplementary materials.
- 3. Statistical analyses
- The results are summarized as median (quartile) or number (%), as appropriate. We also tested whether opinions differed by sub-specialty or year of experience. t-, chi-square, or Fisher’s exact tests were used for comparisons, as appropriate. P <0.05 was considered significant.
METHODS
- 1. Assessment of status by KLCA members
- Ninety-nine percent of the participating KLCA members agreed that hepatocellular carcinoma (HCC) surveillance in high-risk patients lowers the risk of HCC-related death (Fig. 1). Regarding the NLCSP in Korea, 99% reported that they knew the current NLCSP. When asked about the NLCSP’s role in Korea, 95% responded that the NLCSP contributes to lowering the risk of HCC-related deaths in Korea (Fig. 1). When asked, “How many points do you give the NLCSP?” (lowest 0 points-highest 10 points), the median point was 7 (range, 2-10). The mean points were lower in members with more than 10 years of experience than that in those with less than 10 years of experience (6.96±1.51 vs. 7.76±1.10, P =0.009). When the analysis was limited to 72 hepatologists, 30 had less than 10 years of experience, while 42 had more than 10 years of experience. Hepatologists with more experience rated the NLCSP more negatively (Supplementary Table 1). However, there were no significant differences in opinions on the target group, target group identification process, or priority for improvement except for agreement rates on the starting age for surveillance and the reasons why the selection process needs improvement (Supplementary Table 1).
- 2. Assessment of target groups
- Currently, the NLCSP offers liver US and serum AFP measurement every six months for high-risk individuals (adults older than 40 years with cirrhosis, chronic hepatitis B virus [HBV], or HCV infection, according to https://www.g-health.kr/portal/index.do [accessed December 21, 2019]). When asked, 5.6%, 57.8%, 25.6%, and 1.1% of survey participants responded that the current target populations were very appropriate, appropriate, inappropriate, and very inappropriate target populations for the NLCSP, respectively. Specifically, 22.2% responded that cirrhosis over 40 years of age was an inappropriate target, 20% responded that chronic HBV infection over 40 years of age was inappropriate, and 30% responded that chronic HCV infection over 40 years of age was an inappropriate target. Six opinions on ‘HCC surveillance target’ were selected by the project committee members and asked for agreement. The agreement rates ranged from 8.9% to 82.2% for each question (Table 2).
- 3. Assessment of the target group identification process
- In the NLCSP, the target population (high-risk individuals) is identified by reviewing National Health Insurance Service (NHIS) claims data. The NHIS in Korea is a single-payer universal health system that maintains claims data on all reimbursed inpatient and outpatient visits, procedures, and prescriptions. These claims data are coded using the International Classification of Diseases, Tenth Revision (ICD-10) and the Korean Drug and Anatomical Therapeutic Chemical Codes. The NLCSP identifies high-risk individuals, defined as having NHIS claims for the past two years with the disease classification codes shown in Table 3.
- Fifty percent of survey participants responded that they were aware of the target population selection process of the NLCSP. When asked whether this selection process needed improvement, 20%, 67.8%, and 10% responded that improvement was very necessary, necessary, and not necessary, respectively, while two participants (2.2%) did not respond. When asked ‘Why does the selection process need improvement?’, 58.9% agreed that the screening targets identified by disease classification codes may not match true surveillance targets, 17.8% agreed that there was a privacy violation problem and 78.9% agreed that the current process identifies individuals who use medical services, paradoxically missing individuals who do not. Three opinions on ‘How the target population selection process can be improved’ were selected by the project committee members and asked for agreement. The agreement rates ranged from 40.0% to 50.0% for each question (Table 2).
- 4. Assessment of priorities for improvement
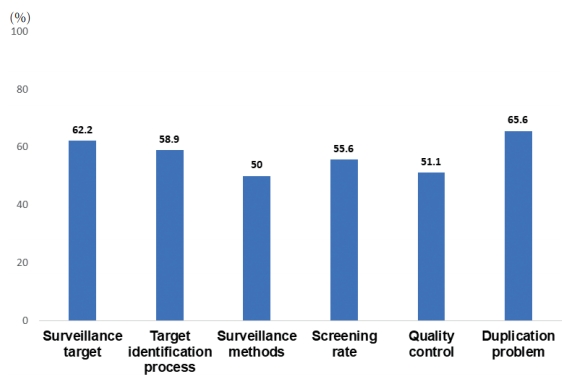
- When asked for the foremost priority for improvement, solving duplication issues between the NLCSP and private clinic HCC screening practices were the most commonly selected choices (23.3%), followed by improving surveillance methods (e.g., allowing the use of computed tomography or magnetic resonance imaging, allowing the use of other tumor markers, and allowing intensive surveillance at short-term intervals) (21.1%), improving low screening rates (16.7%), improving the target identification process (14.4%), improving the definition of the target population (13.3%), and improving the quality of the NLCSP (8.9%) (Fig. 2). For each specific area, 50.0% to 65.6% of the survey participants responded that improvement was needed (Fig. 3). There were no differences in the foremost priorities according to the respondents’ years of experience, sub-specialties, or workplace (data not shown).
RESULTS
- In this survey, 99% of survey participants agreed that HCC surveillance in high-risk patients could lower the risk of HCC-related death (71% strongly agreed; 28% agreed). Ninety-five percent also agreed that the NLCSP helps to reduce the HCC mortality rate in Korea. Yet, only about onequarter (27%) answered that the NLCSP is very contributing, while two-thirds of the survey participants (68%) rated the NLCSP as contributing to the reduction only to some extent. There was a large gap between belief (71% strongly agreed that HCC surveillance can lower the risk of HCC-related death) and the actual role of the NLCSP (27% rated that the NLCSP highly contributed to reducing HCC mortality in Korea). The median score of the current NLCSP was 7 points (max 10 points). It suggests that the survey participants think HCC surveillance can reduce the risk of HCC-related death, but that the NLCSP is not playing a sufficient role.
- Several issues have been raised regarding the NLCSP in Korea. A lowered mortality risk (hazard ratio, 0.78; 95% confidence interval, 0.76 to 0.80) was recently reported among patients who participated in the NLCSP once within two years prior to the diagnosis of liver cancer compared to those who did not participate in the NLCSP [8], indicating the potential role of the NLCSP in reducing HCC-related mortality. However, other studies reported poor efficacy of the NLCSP. A study that assessed the 2010 National Cancer Screening Program reported very poor performance of the NLCSP. The positive predictive value was only 5.7% and the sensitivity was 41.3% [9], meaning that the NLCSP program is not very effective despite the tremendous amount of government funding. A study from a single healthcare center reported that many (46% of NLCSP participants) individuals were inaccurately identified as the NLCSP target population [10]. Another cohort study of 541 chronic hepatitis B patients who participated in the NLCSP reported that tumors were detected in only nine of 16 patients (56.3%) under the NLCSP, whereas tumors were detected in seven of 16 patients (43.7%) by computed tomography or magnetic resonance imaging evaluation outside of the NLCSP [11]. The participant rates for the NLCSP increased steadily from 13.2% in 2003 to 39.5% in 2012 [4] but were lowest (33.6%) compared to other cancers (stomach, colorectal, breast and cervical cancer; 73.6%, 55.6%, 59.7% and 67.0%, respectively) in 2013 [12]. The quality of US screening was demonstrated to be sub-optimal in 143/685 hospitals (20.8%) and 645/1,985 (32.5%) private clinics that failed to pass quality assurance evaluation for liver cancer screening by US [13]. In this survey study, most of the survey participants (87.8%) responded that the current target group identification process requires improvement. Most (78.9%) were also concerned about missing surveillance targets by using disease classification codes of NHIS claims data for identification. In this survey, more than 50% of respondents agreed that areas of the NLCSP need improvement (Fig. 3). It is clear that the NLCSP warrants further improvement.
- Regarding suggestions to improve the NLCSP, a high rate of agreement was observed on two surveillance targets. The majority of respondents (82.2%) agreed that patients with liver cirrhosis should be included regardless of age. In fact, the Korean guideline for HCC surveillance recommends surveillance for those with HBV or HCV infection or cirrhosis from 40 years of age or at the time of cirrhosis diagnosis [14]. The reason why the NLCSP is provided for those only aged over 40 years remains unclear and cirrhosis patients should be included in the NLCSP regardless of age. Most (72.2%) participants also agreed that the NLCSP should include chronic hepatitis patients with advanced fibrosis as these patients are at high risk for HCC [15,16]. Inclusion of these patients should be considered for the first candidate group when expanding the NLCSP. In terms of the target group identification process, none of the three suggestions for improving the surveillance target selection process achieved high agreement rates (Table 2). Thus, further studies are needed to determine how to improve the surveillance target selection process. Opinions were diverse regarding the foremost priority for improvement (Fig. 2), with solving duplication issues between the NLCSP and private clinic HCC screening practices receiving the most choices (23.3%). Efforts to improve the NLCSP are urgently needed.
- This study had some limitations. The fairness of survey items and evaluation methods has not been validated by professional survey researchers and not guided by a theoretical framework. The survey form was sent to 735 KLCA members; however, the overall response rate was low (12.2%) and may not represent the opinions of all KLCA members. Specifically, the respondents included 72 of 368 hepatologists (19.6%); 11 of 127 surgeons (8.7%); 3 of 143 radiologists (2.1%); and 4 of 97 radiation oncologists, pathologists, and other specialties (4.1%). Most of the survey participants (87.8%) worked in university hospitals and hepatologists comprised 80.0% of participants. The NLCSP is widely practiced by physicians in many specialties and is not only performed in university hospitals. Opinions from other practice areas are needed. The survey was conducted in Korean; hence, its generalizability to other countries with different cultural and medical backgrounds is limited. The survey asked for expert opinions without providing detailed data on the NLCSP in Korea (program cost, HCC diagnosis rate, true positive rate, false-positive rate, false-negative rate, participation rate, etc.). Thus, the survey participants may have under- or over-estimated the actual contributions of the NLCSP in reducing liver cancer mortality in Korea. The strength of this survey is that it is the first structured and organized report on the views of liver cancer specialists of the NLCSP in Korea.
- In summary, this survey found generally positive attitudes among liver cancer specialists regarding the role of the NLCSP. However, most of the survey participants rated the NCLSP as needing improvement. The findings from this survey can provide relevant information and may help future health policy decisions.
DISCUSSION
Supplementary Materials
Supplementary Table 1.


- 1. Kim BH, Park JW. Epidemiology of liver cancer in South Korea. Clin Mol Hepatol 2018;24:1−9.ArticlePubMedPDF
- 2. Kweon SS. Epidemiology of liver cancer in Korea. J Korean Med Assoc 2019;62:416−423.Article
- 3. Yoo KY. Cancer control activities in the Republic of Korea. Jpn J Clin Oncol 2008;38:327−333.ArticlePubMedPDF
- 4. Suh M, Song S, Cho HN, Park B, Jun JK, Choi E, et al. Trends in Participation Rates for the National Cancer Screening Program in Korea, 2002-2012. Cancer Res Treat 2017;49:798−806.ArticlePubMedPDF
- 5. Kim Y, Jun JK, Choi KS, Lee HY, Park EC. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev 2011;12:725−730.PubMed
- 6. Korean Liver Cancer Association, National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver 2019;13:227−299.ArticlePubMedPMC
- 7. Jung KW, Won YJ, Kong HJ, Oh CM, Shin A, Lee JS. Survival of Korean adult cancer patients by stage at diagnosis, 2006-2010: national cancer registry study. Cancer Res Treat 2013;45:162−171.ArticlePubMedPMCPDF
- 8. Kwon JW, Tchoe HJ, Lee J, Suh JK, Lee JH, Shin S. The impact of national surveillance for liver cancer: Results from Real-World Setting in Korea. Gut Liver 2020;14:108−116.ArticlePubMedPDF
- 9. Jung M. National Cancer Screening Programs and evidence-based healthcare policy in South Korea. Health policy 2015;119:26−32.ArticlePubMed
- 10. Shim JJ, Park HJ, Kim JW, Hwang EJ, Lee CK, Jang JY, et al. The Korean National Liver Cancer Surveillance Program: Experience of a Single Healthcare Center in 2011. Korean J Med 2013;84:672−680.ArticlePDF
- 11. Choi IS, Oh CH, Park SY, Ahn SE, Park SJ, Choi HR, et al. Incidence of primary liver cancer in subjects with chronic hepatitis B in Korean National Liver Cancer Screening Program. J Liver Cancer 2017;17:136−143.Article
- 12. Suh M, Choi KS, Park B, Lee YY, Jun JK, Lee DH, et al. Trends in Cancer Screening Rates among Korean Men and Women: Results of the Korean National Cancer Screening Survey, 2004-2013. Cancer Res Treat 2016;48:1−10.ArticlePubMedPDF
- 13. Choi MH, Jung SE, Choi JI, Jeong WK, Kim HC, Kim Y, et al. Quality management of ultrasound surveillance for hepatocellular carcinoma under the Korean National Cancer Screening Program. J Ultrasound Med 2018;37:245−254.ArticlePubMed
- 14. Kim DY, Kim HJ, Jeong SE, Kim SG, Kim HJ, Sinn DH, et al. The Korean guideline for hepatocellular carcinoma surveillance. J Korean Med Assoc 2015;58:385−397.Article
- 15. Na SK, Song BC. Development and surveillance of hepatocellular carcinoma in patients with sustained virologic response after antiviral therapy for chronic hepatitis C. Clin Mol Hepatol 2019;25:234−244.ArticlePubMedPMCPDF
- 16. Zhang X, Wong GL, Wong VW. Application of transient elastography in nonalcoholic fatty liver disease. Clin Mol Hepatol 2019;Nov 8 . doi: 10.3350/cmh.2019.0001n. [Epub ahead of print].ArticlePDF
References
Figure & Data
References
Citations

- Potential role of Fibrosis‐4 score in hepatocellular carcinoma screening: The Kangbuk Samsung Health Study
Sujeong Shin, Won Sohn, Yoosoo Chang, Yoosun Cho, Min‐Jung Kwon, Sarah H. Wild, Christopher D. Byrne, Seungho Ryu
Hepatology Research.2024;[Epub] CrossRef - Clinical practice guideline and real-life practice in hepatocellular carcinoma: A Korean perspective
Myung Ji Goh, Dong Hyun Sinn, Jong Man Kim, Min Woo Lee, Dong Ho Hyun, Jeong Il Yu, Jung Yong Hong, Moon Seok Choi
Clinical and Molecular Hepatology.2023; 29(2): 197. CrossRef - Current status of ultrasonography in national cancer surveillance program for hepatocellular carcinoma in South Korea: a large-scale multicenter study
Sun Hong Yoo, Soon Sun Kim, Sang Gyune Kim, Jung Hyun Kwon, Han-Ah Lee, Yeon Seok Seo, Young Kul Jung, Hyung Joon Yim, Do Seon Song, Seong Hee Kang, Moon Young Kim, Young-Hwan Ahn, Jieun Han, Young Seok Kim, Young Chang, Soung Won Jeong, Jae Young Jang, J
Journal of Liver Cancer.2023; 23(1): 189. CrossRef - Selecting the Target Population for Screening of Hepatic Fibrosis in Primary Care Centers in Korea
Huiyul Park, Eileen L. Yoon, Mimi Kim, Seon Cho, Jung-Hwan Kim, Dae Won Jun, Eun-Hee Nah
Journal of Clinical Medicine.2022; 11(6): 1474. CrossRef - Fibrosis Burden of Missed and Added Populations According to the New Definition of Metabolic Dysfunction-Associated Fatty Liver
Huiyul Park, Eileen L. Yoon, Mimi Kim, Jung-Hwan Kim, Seon Cho, Dae Won Jun, Eun-Hee Nah
Journal of Clinical Medicine.2021; 10(19): 4625. CrossRef

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION
 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation

 Follow JLC on Twitter
Follow JLC on Twitter