Search
- Page Path
- HOME > Search
Review Article
- Current perspectives on radiotherapy in hepatocellular carcinoma management: a comprehensive review
- Dowook Kim, Jun-Sang Kim
- J Liver Cancer. 2024;24(1):33-46. Published online March 25, 2024
- DOI: https://doi.org/10.17998/jlc.2024.02.26
- 494 Views
- 47 Downloads
-
 Abstract
Abstract
 PDF
PDF - This review examines the transformative role of external beam radiotherapy (EBRT) in managing hepatocellular carcinoma (HCC), spotlighting the progression from traditional EBRT techniques to advanced modalities like intensity-modulated radiotherapy (RT), stereotactic body RT (SBRT), and innovative particle therapy, including proton beam therapy and carbon ion RT. These advancements have significantly improved the precision and efficacy of RT, marking a paradigm shift in the multimodal management of HCC, particularly in addressing complex cases and enhancing local tumor control. The review underscores the synergistic potential of integrating RT with other treatments like transarterial chemoembolization, systemic therapies such as sorafenib, and emerging immunotherapies, illustrating enhanced survival and disease control outcomes. The efficacy of RT is addressed for challenging conditions, including advanced HCC with macrovascular invasion, and RT modalities, like SBRT, are compared against traditional treatments like radiofrequency ablation for early-stage HCC. Additionally, the review accentuates the encouraging outcomes of particle therapy in enhancing local control and survival rates, minimizing treatment-related toxicity, and advocating for continued research and clinical trials. In conclusion, the integration of RT into multimodal HCC treatment strategies, coupled with the emergence of particle therapy, is crucial for advancing oncologic management, emphasizing the need for relentless innovation and personalized treatment approaches.

Original Articles
- Liver resection in selective hepatocellular carcinoma with Vp3 or Vp4 portal vein tumor thrombosis improves prognosis
- Manuel Lim, Jongman Kim, Jinsoo Rhu, Gyu-Seong Choi, Jae-Won Joh
- J Liver Cancer. 2024;24(1):102-112. Published online February 14, 2024
- DOI: https://doi.org/10.17998/jlc.2024.01.31
- 402 Views
- 38 Downloads
-
 Abstract
Abstract
 PDF
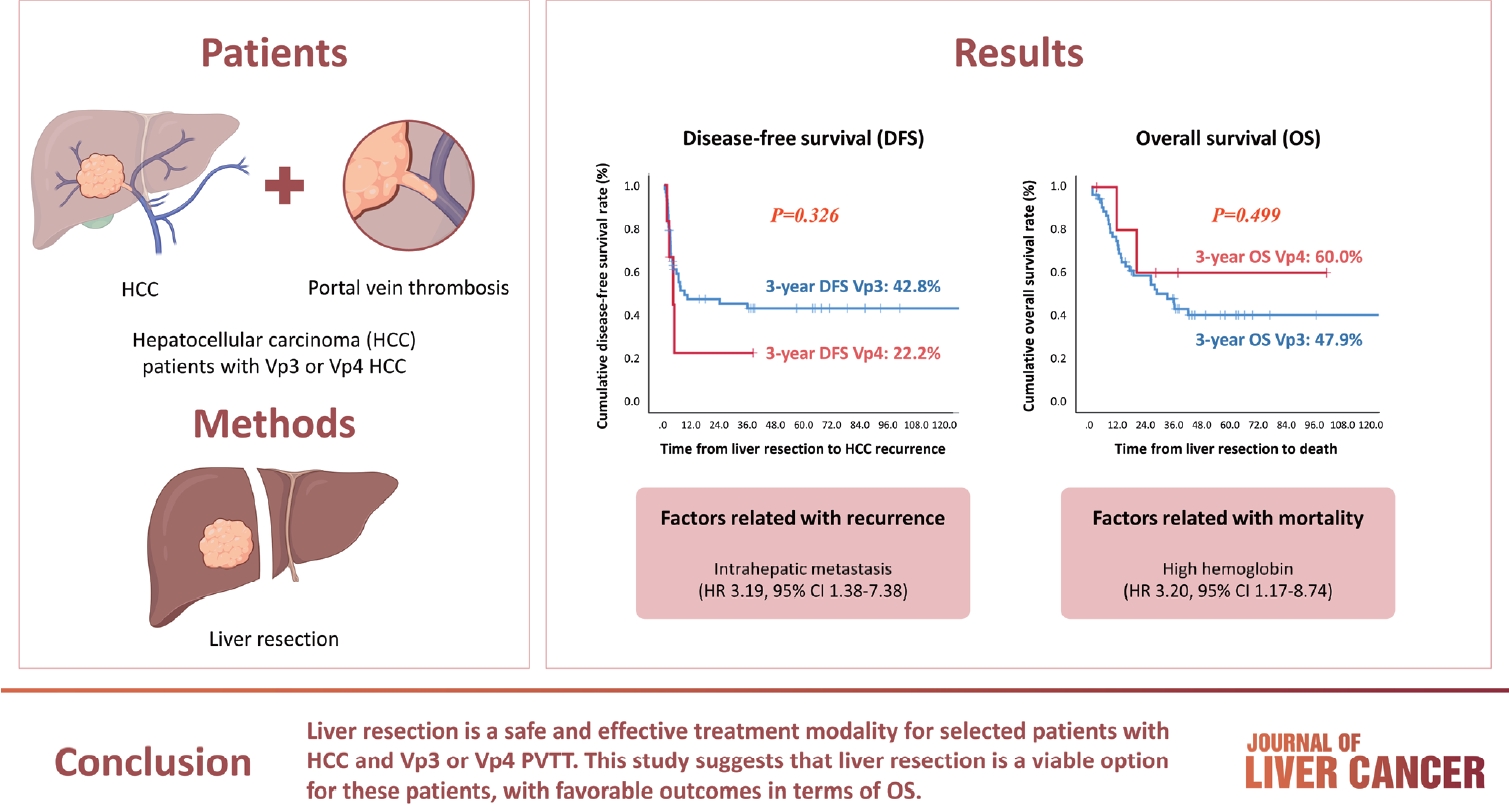
PDF - Background/Aim
Hepatocellular carcinoma (HCC) tumor thrombi located in the first branch of the portal vein (Vp3) or in the main portal trunk (Vp4) are associated with poor prognosis. This study aimed to investigate the clinicopathological characteristics and risk factors for HCC recurrence and mortality following liver resection (LR) in patients with Vp3 or Vp4 HCC.
Methods
The study included 64 patients who underwent LR for HCC with Vp3 or Vp4 portal vein tumor thrombosis (PVTT).
Results
Fifty-eight patients (90.6%) had Vp3 PVTT, whereas the remaining six patients exhibited Vp4 PVTT. The median tumor size measured 8 cm, with approximately 36% of patients presented with multiple tumors. Fifty-four patients (84.4%) underwent open LR, whereas 10 patients underwent laparoscopic LR. In the Vp4 cases, combined LR and tumor thrombectomy were performed. The 3-year cumulative disease-free survival rate was 42.8% for the Vp3 group and 22.2% for the Vp4 group. The overall survival (OS) rate at 3 years was 47.9% for the Vp3 group and 60.0% for the Vp4 group. Intrahepatic metastasis has been identified as an important contributor to HCC recurrence. High hemoglobin levels are associated with high mortality.
Conclusion
LR is a safe and effective treatment modality for selected patients with Vp3 or Vp4 HCC PVTT. This suggests that LR is a viable option for these patients, with favorable outcomes in terms of OS.

- Comparison of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma with portal vein tumor thrombosis
- Jeayeon Park, Yun Bin Lee, Yunmi Ko, Youngsu Park, Hyunjae Shin, Moon Haeng Hur, Min Kyung Park, Dae-Won Lee, Eun Ju Cho, Kyung-Hun Lee, Jeong-Hoon Lee, Su Jong Yu, Tae-Yong Kim, Yoon Jun Kim, Tae-You Kim, Jung-Hwan Yoon
- J Liver Cancer. 2024;24(1):81-91. Published online January 19, 2024
- DOI: https://doi.org/10.17998/jlc.2023.12.25
- 983 Views
- 132 Downloads
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
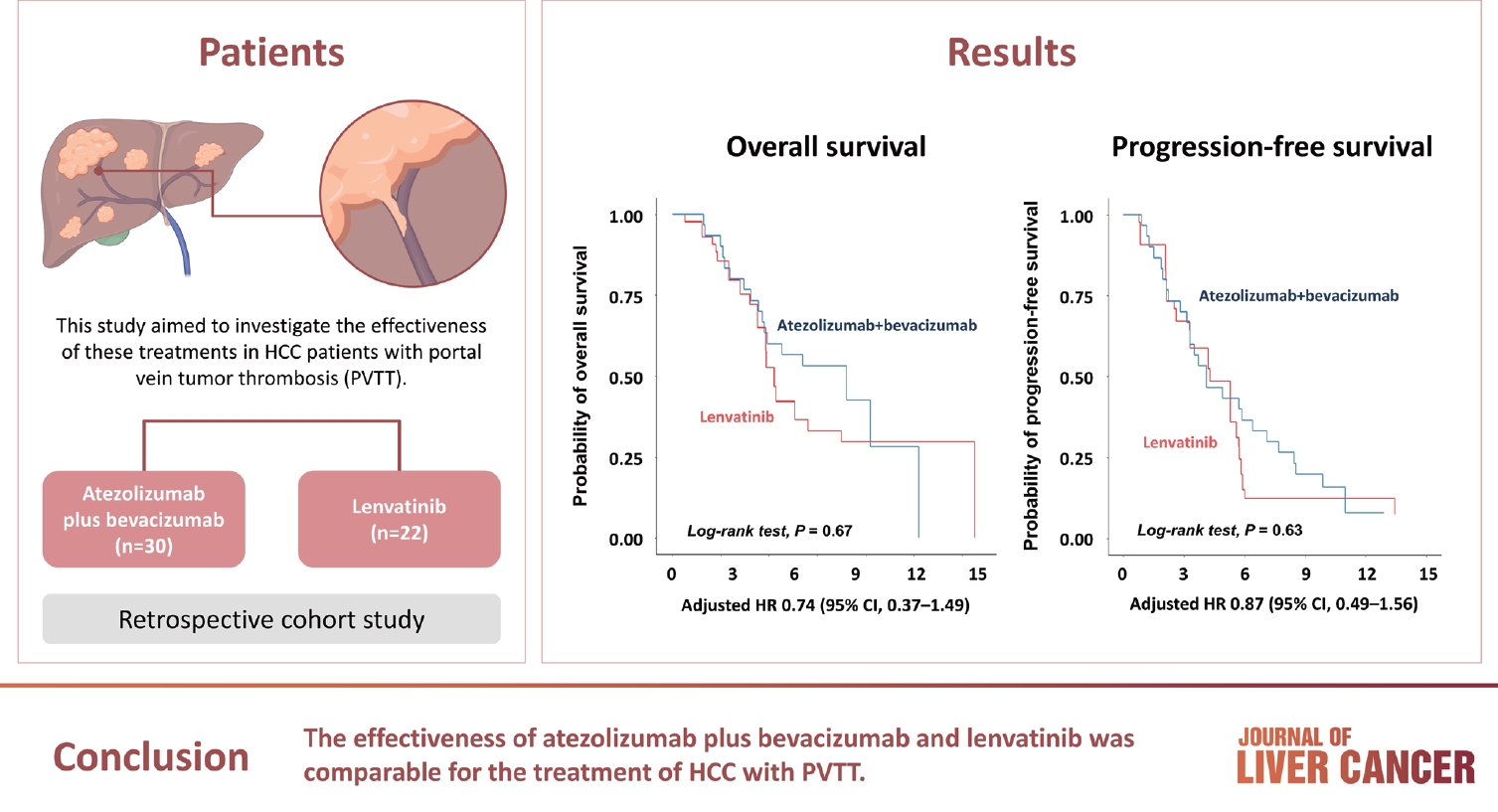
Supplementary Material - Background/Aim
Atezolizumab plus bevacizumab and lenvatinib are currently available as first-line therapy for the treatment of unresectable hepatocellular carcinoma (HCC). However, comparative efficacy studies are still limited. This study aimed to investigate the effectiveness of these treatments in HCC patients with portal vein tumor thrombosis (PVTT).
Methods
We retrospectively included patients who received either atezolizumab plus bevacizumab or lenvatinib as first-line systemic therapy for HCC with PVTT. Primary endpoint was overall survival (OS), and secondary endpoints included progressionfree survival (PFS) and disease control rate (DCR) determined by response evaluation criteria in solid tumors, version 1.1.
Results
A total of 52 patients were included: 30 received atezolizumab plus bevacizumab and 22 received lenvatinib. The median follow-up duration was 6.4 months (interquartile range, 3.9-9.8). The median OS was 10.8 months (95% confidence interval [CI], 5.7 to not estimated) with atezolizumab plus bevacizumab and 5.8 months (95% CI, 4.8 to not estimated) with lenvatinib (P=0.26 by log-rank test). There was no statistically significant difference in OS (adjusted hazard ratio [aHR], 0.71; 95% CI, 0.34-1.49; P=0.37). The median PFS was similar (P=0.63 by log-rank test), with 4.1 months (95% CI, 3.3-7.7) for atezolizumab plus bevacizumab and 4.3 months (95% CI, 2.6-5.8) for lenvatinib (aHR, 0.93; 95% CI, 0.51-1.69; P=0.80). HRs were similar after inverse probability treatment weighting. The DCRs were 23.3% and 18.2% in patients receiving atezolizumab plus bevacizumab and lenvatinib, respectively (P=0.74).
Conclusion
The effectiveness of atezolizumab plus bevacizumab and lenvatinib was comparable for the treatment of HCC with PVTT.

Review Articles
- Complications of immunotherapy in advanced hepatocellular carcinoma
- Young-Gi Song, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
- J Liver Cancer. 2024;24(1):9-16. Published online November 29, 2023
- DOI: https://doi.org/10.17998/jlc.2023.11.21
- 973 Views
- 75 Downloads
-
 Abstract
Abstract
 PDF
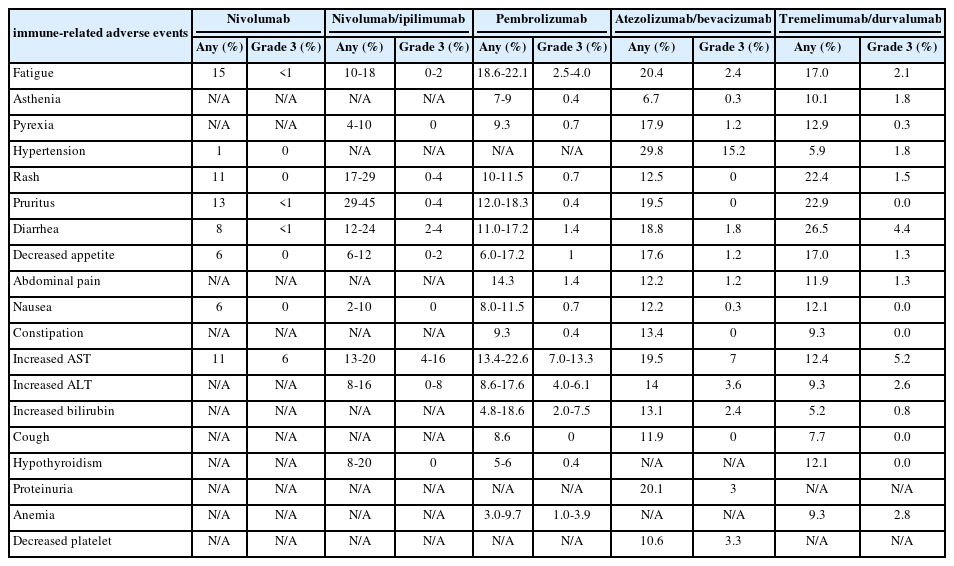
PDF - Immune checkpoint inhibitors (ICIs) are highly effective in cancer treatment. However, the risks associated with the treatment must be carefully balanced against the therapeutic benefits. Immune-related adverse events (irAEs) are generally unpredictable and may persist over an extended period. In this review, we analyzed common irAEs reported in highly cited original articles and systematic reviews. The prevalent adverse reactions include fatigue, pyrexia, rash, pruritus, diarrhea, decreased appetite, nausea, abdominal pain, constipation, hepatitis, and hypothyroidism. Therefore, it is crucial to conduct evaluations not only of gastrointestinal organs but also of cardiac, neurologic, endocrine (including the frequently affected thyroid), and ophthalmic systems before commencing ICIs. This review further explores commonly reported types of irAEs, specific irAEs associated with each ICI agent, rare yet potentially fatal irAEs, and available treatment options for managing them.

- A multidisciplinary approach with immunotherapies for advanced hepatocellular carcinoma
- Yu Rim Lee
- J Liver Cancer. 2023;23(2):316-329. Published online September 22, 2023
- DOI: https://doi.org/10.17998/jlc.2023.09.04

- 1,548 Views
- 101 Downloads
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF - Hepatocellular carcinoma (HCC) is a highly aggressive disease that is usually diagnosed at an advanced stage. Advanced HCC has limited treatment options and often has a poor prognosis. For the past decade, tyrosine kinase inhibitors have been the only treatments approved for advanced HCC that have shown overall survival (OS) benefits; however, but their clinical efficacy has been limited. Recent trials have demonstrated promising advancements in survival outcomes through immunotherapy-based treatments, such as combinations of immune checkpoint inhibitors (ICIs) with other ICIs, antiangiogenic drugs, and locoregional therapies. The atezolizumab-bevacizumab and durvalumab-tremelimumab (STRIDE) regimen has significantly improved survival rates as a first-line treatment and has become the new standard of care. Therefore, combined treatments for advanced HCC can result in better treatment outcomes owing to their synergistic effects, which requires a multidisciplinary approach. Ongoing studies are examining other therapeutic innovations that can improve disease control and OS rates. Despite improvements in the treatment of advanced HCC, further studies on the optimal treatment selection and sequences, biomarker identification, combination approaches with other therapies, and development of novel immunotherapy agents are required. This review presents the current treatment options and clinical data of the ICI-based combination immunotherapies for advanced HCC from a multidisciplinary perspective.
-
Citations
Citations to this article as recorded by- Reduced-Dose or Discontinuation of Bevacizumab Might Be Considered after Variceal Bleeding in Patients with Hepatocellular Carcinoma Receiving Atezolizumab/Bevacizumab: Case Reports
Kyeong-Min Yeom, Young-Gi Song, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim
Medicina.2024; 60(1): 157. CrossRef - Hepatocellular Carcinoma: Advances in Systemic Therapy
Insija Ilyas Selene, Merve Ozen, Reema A. Patel
Seminars in Interventional Radiology.2024; 41(01): 056. CrossRef
- Reduced-Dose or Discontinuation of Bevacizumab Might Be Considered after Variceal Bleeding in Patients with Hepatocellular Carcinoma Receiving Atezolizumab/Bevacizumab: Case Reports

Original Article
- The efficacy of treatment for hepatocellular carcinoma in elderly patients
- Han Ah Lee, Sangheun Lee, Hae Lim Lee, Jeong Eun Song, Dong Hyeon Lee, Sojung Han, Ju Hyun Shim, Bo Hyun Kim, Jong Young Choi, Hyunchul Rhim, Do Young Kim
- J Liver Cancer. 2023;23(2):362-376. Published online September 14, 2023
- DOI: https://doi.org/10.17998/jlc.2023.08.03
- 1,306 Views
- 70 Downloads
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background/Aim
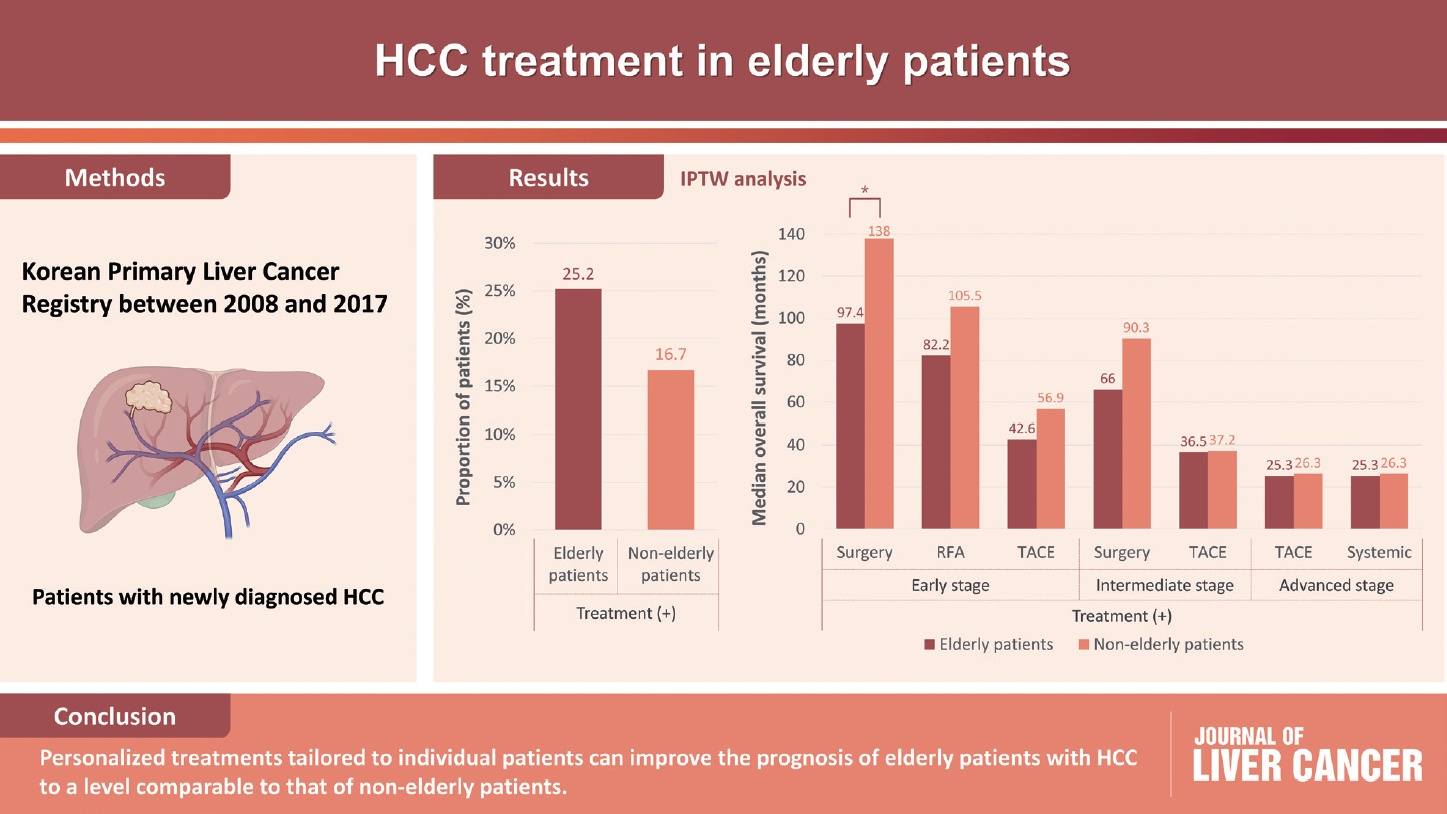
Despite the increasing proportion of elderly patients with hepatocellular carcinoma (HCC) over time, treatment efficacy in this population is not well established.
Methods
Data collected from the Korean Primary Liver Cancer Registry, a representative cohort of patients newly diagnosed with HCC in Korea between 2008 and 2017, were analyzed. Overall survival (OS) according to tumor stage and treatment modality was compared between elderly and non-elderly patients with HCC.
Results
Among 15,186 study patients, 5,829 (38.4%) were elderly. A larger proportion of elderly patients did not receive any treatment for HCC than non-elderly patients (25.2% vs. 16.7%). However, OS was significantly better in elderly patients who received treatment compared to those who did not (median, 38.6 vs. 22.3 months; P<0.001). In early-stage HCC, surgery yielded significantly lower OS in elderly patients compared to non-elderly patients (median, 97.4 vs. 138.0 months; P<0.001), however, local ablation (median, 82.2 vs. 105.5 months) and transarterial therapy (median, 42.6 vs. 56.9 months) each provided comparable OS between the two groups after inverse probability of treatment weighting (IPTW) analysis (all P>0.05). After IPTW, in intermediate-stage HCC, surgery (median, 66.0 vs. 90.3 months) and transarterial therapy (median, 36.5 vs. 37.2 months), and in advanced-stage HCC, transarterial (median, 25.3 vs. 26.3 months) and systemic therapy (median, 25.3 vs. 26.3 months) yielded comparable OS between the elderly and non-elderly HCC patients (all P>0.05).
Conclusions
Personalized treatments tailored to individual patients can improve the prognosis of elderly patients with HCC to a level comparable to that of non-elderly patients.

Letter to the Editor
- Letter regarding “Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab”
- Sun Hyun Bae, Hee Chul Park
- J Liver Cancer. 2023;23(2):402-404. Published online September 8, 2023
- DOI: https://doi.org/10.17998/jlc.2023.08.18
- 669 Views
- 32 Downloads

Original Articles
- Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab
- Tae Hyun Kim, Bo Hyun Kim, Yu Ri Cho, Young-Hwan Koh, Joong-Won Park
- J Liver Cancer. 2023;23(2):330-340. Published online May 16, 2023
- DOI: https://doi.org/10.17998/jlc.2023.04.14
- 1,756 Views
- 112 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background/Aim
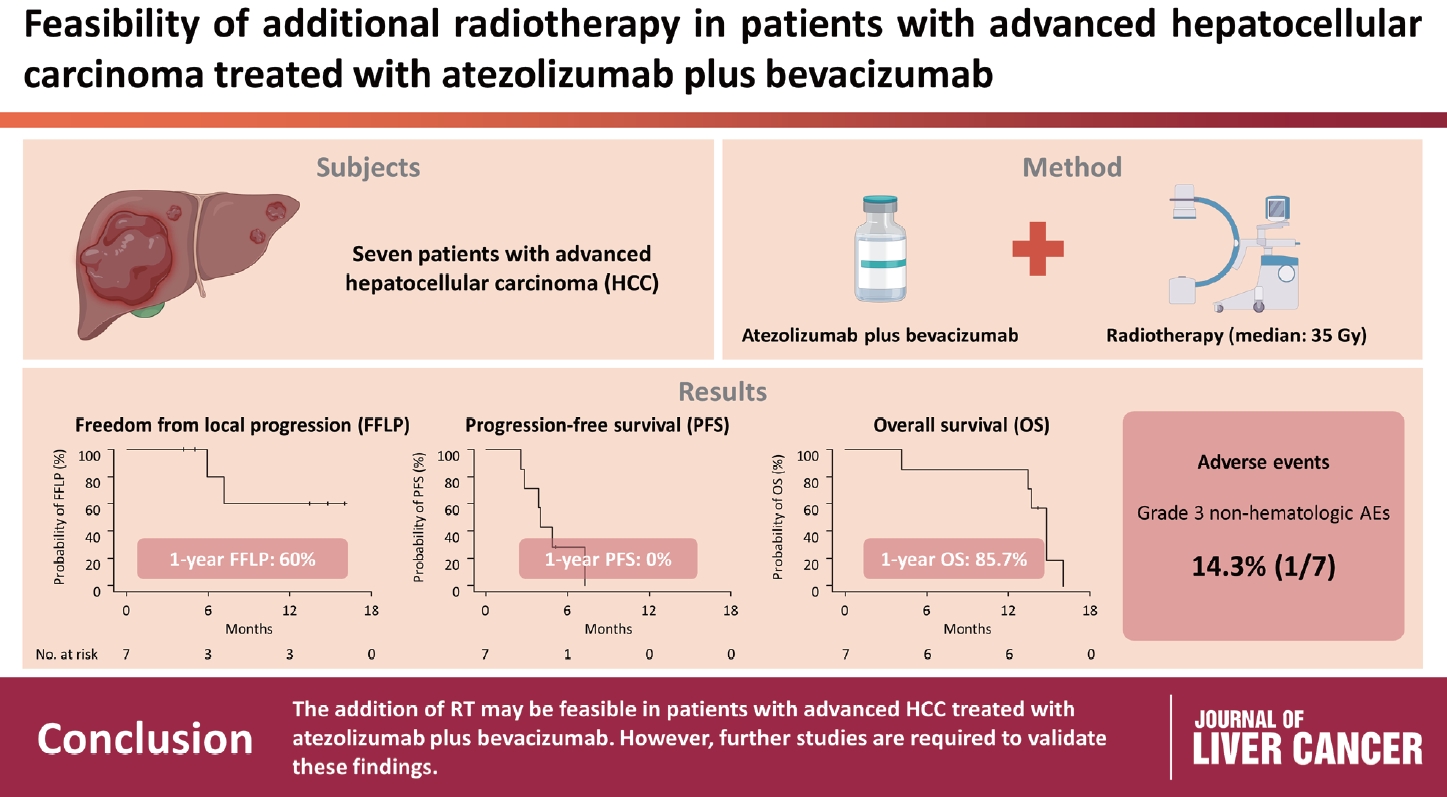
Radiotherapy (RT) is an effective local treatment for hepatocellular carcinoma (HCC). However, whether additional RT is safe and effective in patients with advanced HCC receiving atezolizumab plus bevacizumab remains unclear. This retrospective cohort study aimed to evaluate the feasibility of additional RT in these patients.
Methods
Between March and October 2021, we retrospectively analyzed seven patients with advanced HCC who received RT during treatment with atezolizumab plus bevacizumab. The median prescribed RT dose was 35 Gy (range, 33–66). Freedom from local progression (FFLP), progression-free survival (PFS), and overall survival (OS) after RT were analyzed.
Results
The median follow-up duration after RT was 14.2 months (range, 10.0–18.6). Of the seven patients, disease progression was noted in six (85.7%), the sites of disease progression were local in two (28.6%), intrahepatic in four (57.1%), and extrahepatic in four (57.1%). The median time of FFLP was not reached, and PFS and OS times were 4.0 (95% confidence interval [CI], 3.6–4.5) and 14.8% (95% CI, 12.5–17.2) months, respectively. The 1-year FFLP, PFS, and OS rates were 60% (95% CI, 43.8–76.2), 0%, and 85.7% (95% CI, 75.9–95.5), respectively. Grade 3 or higher hematologic adverse events (AEs) were not observed, but grade 3 nonhematologic AEs unrelated to RT were observed in one patient.
Conclusions
The addition of RT may be feasible in patients with advanced HCC treated with atezolizumab plus bevacizumab. However, further studies are required to validate these findings. -
Citations
Citations to this article as recorded by- Letter regarding “Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab”
Sun Hyun Bae, Hee Chul Park
Journal of Liver Cancer.2023; 23(2): 402. CrossRef
- Letter regarding “Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab”

- Subclassification of advanced-stage hepatocellular carcinoma with macrovascular invasion: combined transarterial chemoembolization and radiotherapy as an alternative first-line treatment
- Sujin Jin, Won-Mook Choi, Ju Hyun Shim, Danbi Lee, Kang Mo Kim, Young-Suk Lim, Han Chu Lee, Jinhong Jung, Sang Min Yoon, Jonggi Choi
- J Liver Cancer. 2023;23(1):177-188. Published online March 23, 2023
- DOI: https://doi.org/10.17998/jlc.2023.03.04

- 1,480 Views
- 88 Downloads
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background/Aim
The Barcelona Clinic Liver Cancer (BCLC) guidelines recommend systemic therapy as the only first-line treatment for patients with BCLC stage C hepatocellular carcinoma (HCC) despite its heterogeneity of disease extent. We aimed to identify patients who might benefit from combined transarterial chemoembolization (TACE) and radiation therapy (RT) by subclassifying BCLC stage C.
Methods
A total of 1,419 treatment-naïve BCLC stage C patients with macrovascular invasion (MVI) who were treated with combined TACE and RT (n=1,115) or systemic treatment (n=304) were analyzed. The primary outcome was overall survival (OS). Factors associated with OS were identified and assigned points by the Cox model. The patients were subclassified into three groups based on these points.
Results
The mean age was 55.4 years, and 87.8% were male. The median OS was 8.3 months. Multivariate analysis revealed a significant association of Child-Pugh B, infiltrative-type tumor or tumor size ≥10 cm, main or bilateral portal vein invasion, and extrahepatic metastasis with poor OS. The sub-classification was categorized into low (point ≤1), intermediate (point=2), and high (point ≥3) risks based on the sum of points (range, 0–4). The OS in the low, intermediate, and high-risk groups was 22.6, 8.2, and 3.8 months, respectively. In the low and intermediate-risk groups, patients treated with combined TACE and RT exhibited significantly longer OS (24.2 and 9.5 months, respectively) than those who received systemic treatment (6.4 and 5.1 months, respectively; P<0.0001).
Conclusions
Combined TACE and RT may be considered as a first-line treatment option for HCC patients with MVI when classified into low- and intermediate-risk groups. -
Citations
Citations to this article as recorded by- Liver resection in selective hepatocellular carcinoma with Vp3 or Vp4 portal vein tumor thrombosis improves prognosis
Manuel Lim, Jongman Kim, Jinsoo Rhu, Gyu-Seong Choi, Jae-Won Joh
Journal of Liver Cancer.2024; 24(1): 102. CrossRef - Comparison of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma with portal vein tumor thrombosis
Jeayeon Park, Yun Bin Lee, Yunmi Ko, Youngsu Park, Hyunjae Shin, Moon Haeng Hur, Min Kyung Park, Dae-Won Lee, Eun Ju Cho, Kyung-Hun Lee, Jeong-Hoon Lee, Su Jong Yu, Tae-Yong Kim, Yoon Jun Kim, Tae-You Kim, Jung-Hwan Yoon
Journal of Liver Cancer.2024; 24(1): 81. CrossRef - How to optimize the treatment strategy for advanced-stage hepatocellular carcinoma with macrovascular invasion
Beom Kyung Kim
Journal of Liver Cancer.2023; 23(1): 121. CrossRef
- Liver resection in selective hepatocellular carcinoma with Vp3 or Vp4 portal vein tumor thrombosis improves prognosis

Case Reports
- Favorable response of hepatocellular carcinoma with portal vein tumor thrombosis after radiotherapy combined with atezolizumab plus bevacizumab
- Yong Tae Kim, Jina Kim, Jinsil Seong
- J Liver Cancer. 2023;23(1):225-229. Published online March 16, 2023
- DOI: https://doi.org/10.17998/jlc.2023.02.27

- 1,067 Views
- 69 Downloads
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF - Recently, the superiority of atezolizumab plus bevacizumab (AteBeva) over sorafenib was proven in the IMbrave150 trial, and AteBeva became the first-line systemic treatment for untreated, unresectable hepatocellular carcinoma (HCC). While the results are encouraging, more than half of patients with advanced HCC are still being treated in a palliative setting. Radiotherapy (RT) is known to induce immunogenic effects that may enhance the therapeutic efficacy of immune checkpoint inhibitors. Herein, we report the case of a patient with advanced HCC with massive portal vein tumor thrombosis treated with a combination of RT and AteBeva, who showed near complete response in tumor thrombosis and favorable response to HCC. Although this is a rare case, it shows the importance of reducing the tumor burden via RT to combination immunotherapy in patients with advanced HCC.
-
Citations
Citations to this article as recorded by- Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab
Tae Hyun Kim, Bo Hyun Kim, Yu Ri Cho, Young-Hwan Koh, Joong-Won Park
Journal of Liver Cancer.2023; 23(2): 330. CrossRef - Carbon Ion Radiotherapy in the Treatment of Hepatocellular Carcinoma
Hwa Kyung Byun, Changhwan Kim, Jinsil Seong
Clinical and Molecular Hepatology.2023; 29(4): 945. CrossRef
- Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab

- A case of nearly complete response in hepatocellular carcinoma with disseminated lung metastasis by combination therapy of nivolumab and ipilimumab after treatment failure of atezolizumab plus bevacizumab
- Hyung Jun Kim, Sang Youn Hwang, Jung Woo Im, Ki Jeong Jeon, Wan Jeon
- J Liver Cancer. 2023;23(1):213-218. Published online March 9, 2023
- DOI: https://doi.org/10.17998/jlc.2023.02.23

- 947 Views
- 65 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
PDF - Recently, the efficacy of immuno-oncologic agents for advanced hepatocellular carcinoma (HCC) has been proven in several trials. In particular, atezolizumab with bevacizumab (AteBeva), as a first-line therapy for advanced HCC, has shown tremendous advances in the IMBrave150 study. However, second or third-line therapy after treatment failure with AteBeva has not been firmly established. Moreover, clinicians have continued their attempts at multidisciplinary treatment that includes other systemic therapy and radiotherapy (RT). Here, we report a case that showed a near complete response (CR) of lung metastasis to nivolumab with ipilimumab therapy after achieving a near CR of intrahepatic tumor using sorafenib and RT in a patient with advanced HCC who had experienced treatment failure of AteBeva.
-
Citations
Citations to this article as recorded by- Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline Update
John D. Gordan, Erin B. Kennedy, Ghassan K. Abou-Alfa, Eliza Beal, Richard S. Finn, Terence P. Gade, Laura Goff, Shilpi Gupta, Jennifer Guy, Hang T. Hoang, Renuka Iyer, Ishmael Jaiyesimi, Minaxi Jhawer, Asha Karippot, Ahmed O. Kaseb, R. Kate Kelley, Jerem
Journal of Clinical Oncology.2024;[Epub] CrossRef
- Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline Update

- A case of successful surgical treatment for peritoneal seeding of hepatocellular carcinoma after radiotherapy and atezolizumab plus bevacizumab combination treatment
- Yuri Cho, Bo Hyun Kim, Tae Hyun Kim, Young Hwan Koh, Joong-Won Park
- J Liver Cancer. 2023;23(1):206-212. Published online February 24, 2023
- DOI: https://doi.org/10.17998/jlc.2023.02.09
- 1,448 Views
- 58 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
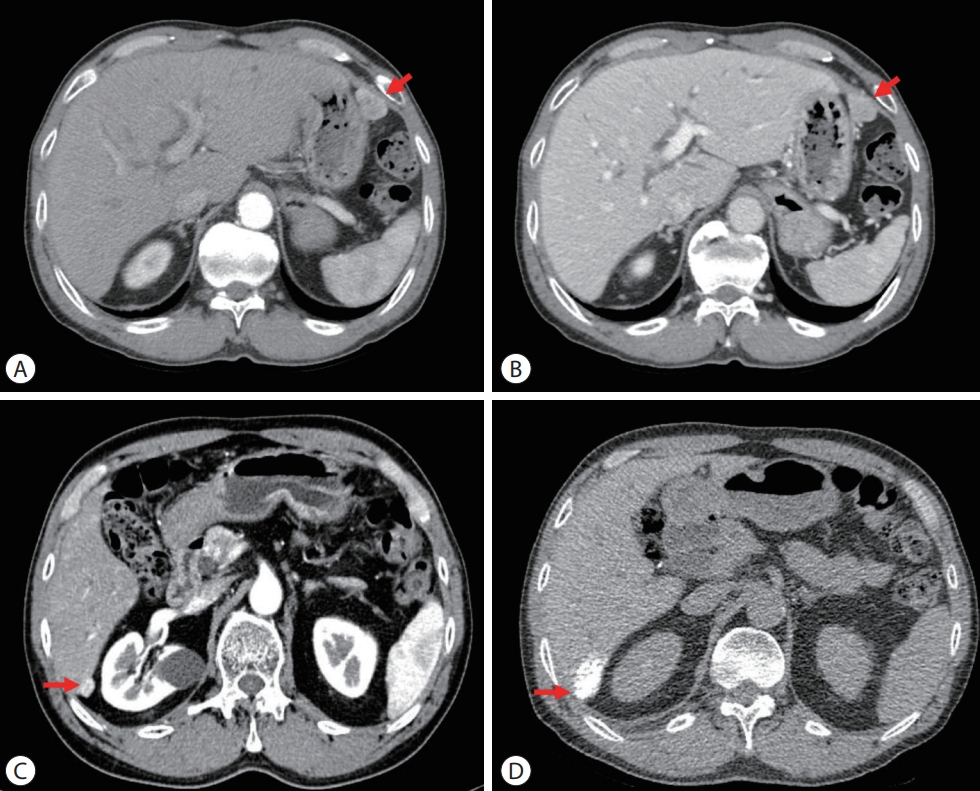
PDF - Peritoneal seeding of hepatocellular carcinoma (HCC) is incurable and has poor prognosis. A 68-year-old man underwent surgical resection for a 3.5 cm single nodular HCC at the tip of segment 3 and transarterial chemoembolization for a 1.5 cm-sized recurrent HCC at the tip of segment 6. 3 months later, an increasing 1 cm pelvic nodule on the rectovesical pouch warranted radiotherapy. Although it stabilized, a new 2.7 cm-sized peritoneal nodule in the right upper quadrant (RUQ) omentum appeared 3.5 years after radiotherapy. Hence, omental mass and small bowel mesentery mass excision were performed. 3 years later, recurrent peritoneal metastases in the RUQ omentum and rectovesical pouch progressed. 33 cycles of atezolizumab and bevacizumab treatment elicited stable disease response. Finally, laparoscopic left pelvic peritonectomy was performed without tumor recurrence. Herein, we present a case of HCC with peritoneal seeding that was successfully treated with surgery after radiotherapy and systemic therapy, leading to complete remission.
-
Citations
Citations to this article as recorded by- Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab
Tae Hyun Kim, Bo Hyun Kim, Yu Ri Cho, Young-Hwan Koh, Joong-Won Park
Journal of Liver Cancer.2023; 23(2): 330. CrossRef
- Feasibility of additional radiotherapy in patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab

Original Article
- Stereotactic body radiation therapy for elderly patients with small hepatocellular carcinoma: a retrospective observational study
- Jeong Yun Jang, Jinhong Jung, Danbi Lee, Ju Hyun Shim, Kang Mo Kim, Young-Suk Lim, Han Chu Lee, Jin-hong Park, Sang Min Yoon
- J Liver Cancer. 2022;22(2):136-145. Published online September 16, 2022
- DOI: https://doi.org/10.17998/jlc.2022.08.18
- 2,950 Views
- 73 Downloads
- 5 Citations
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background/Aim
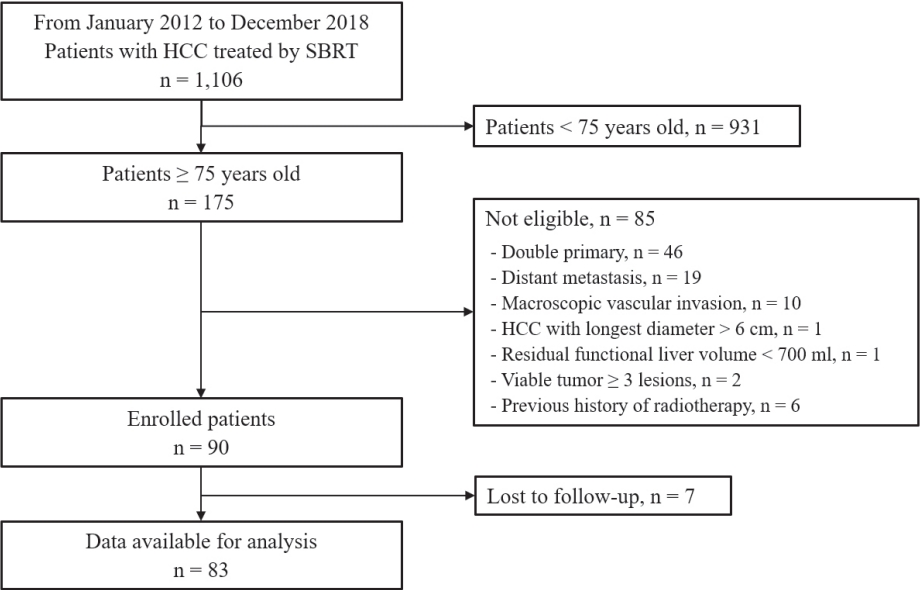
We aimed to investigate the efficacy and safety of stereotactic body radiation therapy (SBRT) in elderly patients with small hepatocellular carcinomas (HCC).
Methods
Eighty-three patients (89 lesions) with HCC who underwent SBRT between January 2012 and December 2018 were reviewed in this retrospective observational study. The key inclusion criteria were as follows: 1) age ≥75 years, 2) contraindications for hepatic resection or percutaneous ablative therapies, 3) no macroscopic vascular invasion, and 4) no extrahepatic metastasis.
Results
The patients were 75-90 years of age, and 49 (59.0%) of them were male. Most patients (94.0%) had an Eastern Cooperative Oncology Group performance status of 0 or 1. Seventy-four patients (89.2%) had Child-Pugh class A hepatic function before SBRT. The median tumor size was 1.6 cm (range, 0.7-3.5). The overall median follow-up period was 34.8 months (range, 7.3-99.3). The 5-year local tumor control rate was 90.1%. The 3-year and 5-year overall survival rate was 57.1% and 40.7%, respectively. Acute toxicity grade ≥3 was observed in three patients (3.6%) with elevated serum hepatic enzymes; however, no patient experienced a worsening of the Child-Pugh score to ≥2 after SBRT. None of the patients developed late toxicity (grade ≥3).
Conclusions
SBRT is a safe treatment option with a high local control rate in elderly patients with small HCC who are not eligible for other curative treatments. -
Citations
Citations to this article as recorded by- Radiofrequency Ablation versus Surgical Resection in Elderly Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
Jeong-Ju Yoo, Sujin Koo, Gi Hong Choi, Min Woo Lee, Seungeun Ryoo, Jungeun Park, Dong Ah Park
Current Oncology.2024; 31(1): 324. CrossRef - Radiotherapy trend in elderly hepatocellular carcinoma: retrospective analysis of patients diagnosed between 2005 and 2017
Bong Kyung Bae, Jeong Il Yu, Hee Chul Park, Myung Ji Goh, Yong-Han Paik
Radiation Oncology Journal.2023; 41(2): 98. CrossRef - Loco-regional therapies competing with radiofrequency ablation in potential indications for hepatocellular carcinoma: a network meta-analysis
Ha Il Kim, Jihyun An, Seungbong Han, Ju Hyun Shim
Clinical and Molecular Hepatology.2023; 29(4): 1013. CrossRef - Has the growing evidence of radiotherapy for hepatocellular carcinoma increased the use of radiotherapy in elderly patients?
Tae Hyun Kim
Radiation Oncology Journal.2023; 41(3): 141. CrossRef - Chronic Liver Disease in the Older Patient—Evaluation and Management
Daniel Anthony DiLeo, Tolga Gidener, Ayse Aytaman
Current Gastroenterology Reports.2023; 25(12): 390. CrossRef
- Radiofrequency Ablation versus Surgical Resection in Elderly Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis

Case Reports
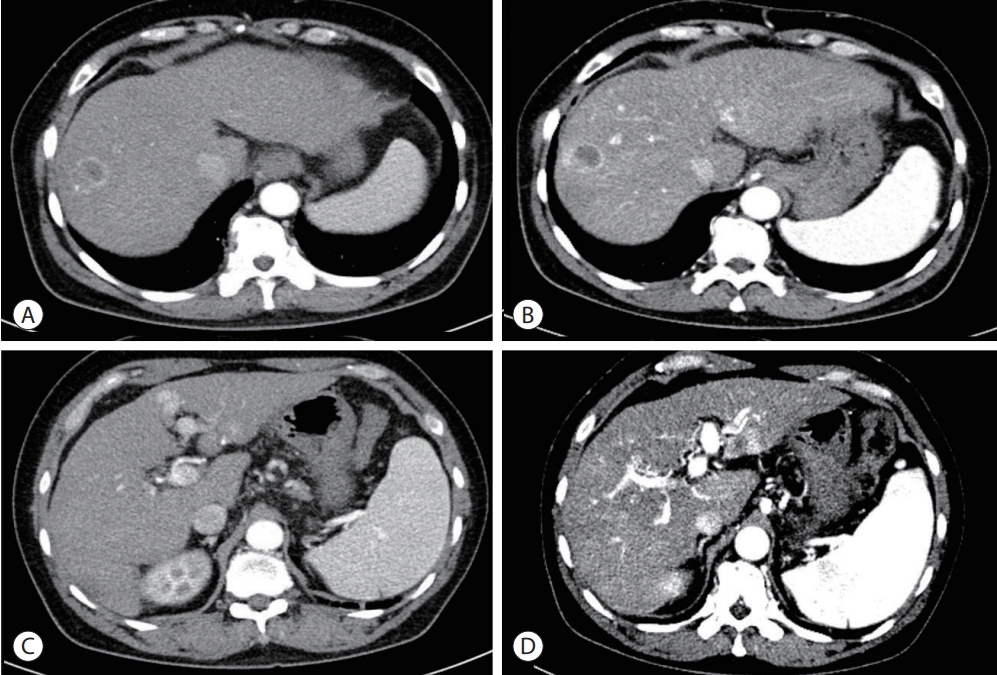
- Novel management of expected post-radiotherapy complications in hepatocellular carcinoma patients: a case report
- Sung Hoon Chang, Tae Suk Kim, Yong Hwan Jeon, Nuri Hyun Jung, Dae Hee Choi
- J Liver Cancer. 2022;22(2):183-187. Published online August 24, 2022
- DOI: https://doi.org/10.17998/jlc.2022.08.03
- 1,653 Views
- 51 Downloads
-
 Abstract
Abstract
 PDF
PDF - In recent years, radiotherapy (RT) has been used to treat hepatocellular carcinoma (HCC) at each stage. This clinical trend has developed with the increasing improvement of RT techniques, which show clinical results comparable to those of other treatment modalities. Intensity-modulated radiotherapy uses a high radiation dose to improve treatment effectiveness. However, the associated radiation toxicity can damage adjacent organs. Radiation-induced gastric damage with gastric ulcers is a complication of RT. This report presents a novel management strategy for preventing post-RT gastric ulcers. We present the case of a 53-year-old male patient diagnosed with HCC, who experienced gastric ulcer after RT. Before the second round of RT, the patient was administered a gas-foaming agent, which was effective in preventing RT complications.

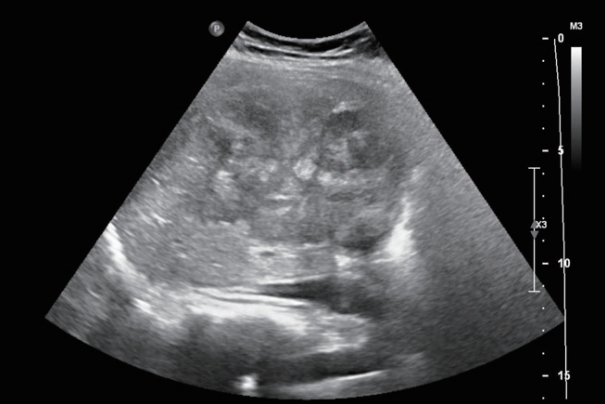
- Fibrolamellar hepatocellular carcinoma that was successfully treated with surgical resection: a case report
- Seong Kyun Na
- J Liver Cancer. 2022;22(2):178-182. Published online June 22, 2022
- DOI: https://doi.org/10.17998/jlc.2022.06.10
- 2,042 Views
- 64 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
PDF - Fibrolamellar hepatocellular carcinoma (FLHCC) is a rare malignant hepatic cancer with characteristics that differ from those of typical hepatocellular carcinoma (HCC). Unlike conventional HCC, FLHCC is common in young patients without any underlying liver disease and is known to be associated with a unique gene mutation. This cancer type is rare in Asia, with only a few cases being reported in Korea. We report a case of FLHCC in a young woman that successfully underwent surgical resection. The efficacy of alternative treatments, such as transarterial chemoembolization or systemic chemotherapies, has not yet been established. To conclude, early diagnosis and appropriate surgical resection are important for the treatment of FLHCC.
-
Citations
Citations to this article as recorded by- Fibrolamellar Hepatocellular Carcinoma (FLHCC) in a Young Patient Presenting With Nausea and Vomiting After a Greasy Meal
Mohamed Ismail , Sahiba Singh, Menna-Allah Elaskandrany , David s Kim, Yazan Abboud, Michael Bebawy, Abena Oduro, Ritik mahaveer Goyal, Omar Mohamed , Weizheng Wang
Cureus.2024;[Epub] CrossRef
- Fibrolamellar Hepatocellular Carcinoma (FLHCC) in a Young Patient Presenting With Nausea and Vomiting After a Greasy Meal


 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION

 First
First Prev
Prev



 Follow JLC on Twitter
Follow JLC on Twitter