Article category
- Page Path
- HOME > Article category > Article category
Original Article
- Gut-microbiome Taxonomic Profiling as Non-invasive Biomarkers for the Early Detection of Alcoholic Hepatocellular Carcinoma
- Jun Seok, Ki Tae Suk
- J Liver Cancer. 2020;20(1):32-40. Published online March 31, 2020
- DOI: https://doi.org/10.17998/jlc.20.1.32
- 5,384 Views
- 233 Downloads
-
 Abstract
Abstract
 PDF
PDF - Background/Aim
s: Hepatocellular carcinoma (HCC) is a prevalent form of primary liver cancer and the fifth leading cause of worldwide cancer mortality. Though early diagnosis of HCC is important, so far lack of effective biomarkers for early diagnosis of HCC has been a problem. In this study, we searched for potential functional biomarkers of alcoholic HCC by using metagenomics approach.
Methods
Between September 2017 and April 2019, normal control (n=44), alcoholic liver cirrhosis (n=44), and alcoholic HCC (n=13) groups were prospectively enrolled and analyzed. Gut microbiota was analyzed using the 16S-based microbiome taxonomic profiling platform of EzBioCloud Apps and analyzing system.
Results
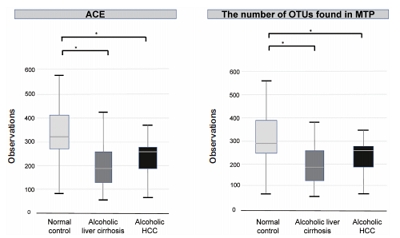
There was a statistically significant difference among groups in diversity (P<0.05). In the comparison of phylum between cirrhosis and HCC, Proteobacteria were increased and Bacteroidetes were decreased. Firmicutes were not significantly different among the three groups. In the taxonomic profiling, relative abundance of Lactobacillus in the cirrhosis and HCC groups showed richness (P<0.05). In the biomarker analysis between cirrhosis and HCC, obiquinome Fe-S protein 3, global nitrogen regulator, Vesicle-associated membrane protein 7, toxin YoeB, peroxisome-assembly ATPase, and nitrogen oxide reductase regulator were differently expressed (P<0.001).
Conclusions
Alcoholic HCC showed different expressions in the stool taxonomy and biomarker compared with that of cirrhosis and control. Therefore, new biomarkers using stool analysis for alcoholic HCC are necessary.

Review Articles
- Liver Magnetic Resonance Imaging for Hepatocellular Carcinoma Surveillance
- So Hyun Park, Bohyun Kim
- J Liver Cancer. 2020;20(1):25-31. Published online March 31, 2020
- DOI: https://doi.org/10.17998/jlc.20.1.25
- 5,599 Views
- 245 Downloads
- 5 Citations
-
 Abstract
Abstract
 PDF
PDF - Hepatocellular carcinoma (HCC) surveillance is recommended when the annual incidence of HCC exceeds 1.5%. In 2018, several international guidelines included alternative surveillance modalities, such as computed tomography and magnetic resonance imaging (MRI), as alternatives for patients with inadequate surveillance with an ultrasound. Currently, abbreviated MRI selectively includes several key sequences and is emerging as an effective tool for HCC surveillance with reduced cost and scan time and the required diagnostic performance. The incidence of HCC substantially impacts the benefits of surveillance in terms of cost-effectiveness. Therefore, we need to individualize imaging surveillance of HCC, tailor screening, and determine risk-stratified strategies. The purpose of this article was to present a brief overview of the diagnostic performance and cost-effectiveness of liver MRI as an HCC surveillance tool.
-
Citations
Citations to this article as recorded by- Adding MRI as a Surveillance Test for Hepatocellular Carcinoma in Patients with Liver Cirrhosis Can Improve Prognosis
Su Jong Yu, Jeong-Ju Yoo, Dong Ho Lee, Su Jin Kim, Eun Ju Cho, Se Hyung Kim, Jeong-Hoon Lee, Yoon Jun Kim, Jeong Min Lee, Jae Young Lee, Jung-Hwan Yoon
Biomedicines.2023; 11(2): 382. CrossRef - MRI features of histologic subtypes of hepatocellular carcinoma: correlation with histologic, genetic, and molecular biologic classification
Ja Kyung Yoon, Jin-Young Choi, Hyungjin Rhee, Young Nyun Park
European Radiology.2022; 32(8): 5119. CrossRef - Imaging features of hepatobiliary MRI and the risk of hepatocellular carcinoma development
Jong-In Chang, Dong Hyun Sinn, Woo Kyoung Jeong, Jeong Ah Hwang, Ho Young Won, Kyunga Kim, Wonseok Kang, Geum-Youn Gwak, Yong-Han Paik, Moon Seok Choi, Joon Hyeok Lee, Kwang Cheol Koh, Seung-Woon Paik
Scandinavian Journal of Gastroenterology.2022; 57(12): 1470. CrossRef - Potential of a Non-Contrast-Enhanced Abbreviated MRI Screening Protocol (NC-AMRI) in High-Risk Patients under Surveillance for HCC
François Willemssen, Quido de Lussanet de la Sablonière, Daniel Bos, Jan IJzermans, Robert De Man, Roy Dwarkasing
Cancers.2022; 14(16): 3961. CrossRef - Prognosis of hepatocellular carcinoma patients diagnosed under regular surveillance: potential implications for surveillance goal
Joo Hye Song, Myung Ji Goh, Yewan Park, Joo Hyun Oh, Wonseok Kang, Dong Hyun Sinn, Geum-Youn Gwak, Yong-Han Paik, Moon Seok Choi, Joon Hyeok Lee, Kwang Cheol Koh, Seung Woon Paik
Scandinavian Journal of Gastroenterology.2021; 56(3): 274. CrossRef
- Adding MRI as a Surveillance Test for Hepatocellular Carcinoma in Patients with Liver Cirrhosis Can Improve Prognosis

- Histopathological Variants of Hepatocellular Carcinomas: an Update According to the 5th Edition of the WHO Classification of Digestive System Tumors
- Haeryoung Kim, Mi Jang, Young Nyun Park
- J Liver Cancer. 2020;20(1):17-24. Published online March 31, 2020
- DOI: https://doi.org/10.17998/jlc.20.1.17

- 14,164 Views
- 1,002 Downloads
- 27 Citations
-
 Abstract
Abstract
 PDF
PDF - Hepatocellular carcinoma (HCC) is heterogeneous in pathogenesis, phenotype and biological behavior. Various histopathological features of HCC had been sporadically described, and with the identification of common molecular alterations of HCC and its genomic landscape over the last decade, morpho-molecular correlation of HCC has become possible. As a result, up to 35% of HCCs can now be classified into histopathological variants, many of which have unique molecular characteristics. This review will provide an introduction to the variously described histopathological variants of HCC in the updated WHO Classification of Digestive System Tumors.
-
Citations
Citations to this article as recorded by- Computational pathology: A survey review and the way forward
Mahdi S. Hosseini, Babak Ehteshami Bejnordi, Vincent Quoc-Huy Trinh, Lyndon Chan, Danial Hasan, Xingwen Li, Stephen Yang, Taehyo Kim, Haochen Zhang, Theodore Wu, Kajanan Chinniah, Sina Maghsoudlou, Ryan Zhang, Jiadai Zhu, Samir Khaki, Andrei Buin, Fatemeh
Journal of Pathology Informatics.2024; 15: 100357. CrossRef - Characterization of lymphocyte‐rich hepatocellular carcinoma and the prognostic role of tertiary lymphoid structures
Bokyung Ahn, Hee‐Sung Ahn, Jinho Shin, Eunsung Jun, Eun‐Young Koh, Yeon‐Mi Ryu, Sang‐Yeob Kim, Chang Ohk Sung, Ju Hyun Shim, JeongYeon Hong, Kyunggon Kim, Hyo Jeong Kang
Liver International.2024; 44(5): 1202. CrossRef - Clinical‐Radiologic Morphology‐Radiomics Model on Gadobenate Dimeglumine‐Enhanced MRI for Identification of Highly Aggressive Hepatocellular Carcinoma: Temporal Validation and Multiscanner Validation
Wanjing Zheng, Xiaodan Chen, Meilian Xiong, Yu Zhang, Yang Song, Dairong Cao
Journal of Magnetic Resonance Imaging.2024;[Epub] CrossRef - Diagnostic Model for Proliferative HCC Using LI‐RADS: Assessing Therapeutic Outcomes in Hepatectomy and TKI‐ICI Combination
Mengtian Lu, Zuyi Yan, Qi Qu, Guodong Zhu, Lei Xu, Maotong Liu, Jifeng Jiang, Chunyan Gu, Ying Chen, Tao Zhang, Xueqin Zhang
Journal of Magnetic Resonance Imaging.2024;[Epub] CrossRef - Low-Baseline PD1+ Granulocytes Predict Responses to Atezolizumab–Bevacizumab in Hepatocellular Carcinoma
Catia Giovannini, Fabrizia Suzzi, Francesco Tovoli, Mariangela Bruccoleri, Mariarosaria Marseglia, Eleonora Alimenti, Francesca Fornari, Massimo Iavarone, Fabio Piscaglia, Laura Gramantieri
Cancers.2023; 15(6): 1661. CrossRef - Non-alcoholic fatty liver disease: the pathologist’s perspective
Wei-Qiang Leow, Anthony Wing-Hung Chan, Paulo Giovanni L. Mendoza, Regina Lo, Kihan Yap, Haeryoung Kim
Clinical and Molecular Hepatology.2023; 29(Suppl): S302. CrossRef - 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma
Journal of Liver Cancer.2023; 23(1): 1. CrossRef - Three-dimensional multifrequency magnetic resonance elastography improves preoperative assessment of proliferative hepatocellular carcinoma
Guixue Liu, Di Ma, Huafeng Wang, Jiahao Zhou, Zhehan Shen, Yuchen Yang, Yongjun Chen, Ingolf Sack, Jing Guo, Ruokun Li, Fuhua Yan
Insights into Imaging.2023;[Epub] CrossRef - Lymphocyte-Rich Hepatocellular Carcinoma with Multiple Lymphadenopathy and Positive Epstein–Barr Virus Encoding Region
Pin-Yi Wang, Yu-Hsuan Kuo, Ming-Jen Sheu, Hsing-Tao Kuo, Wen-Ying Lee, Yu-Ting Kuo, Su-Hung Wang, Zu-Yau Lin
Case Reports in Hepatology.2023; 2023: 1. CrossRef - Genome-Wide Extrachromosomal Circular DNA Profiling of Paired Hepatocellular Carcinoma and Adjacent Liver Tissues
Jianyu Ye, Peixin Huang, Kewei Ma, Zixin Zhao, Ting Hua, Wenjing Zai, Jieliang Chen, Xiutao Fu
Cancers.2023; 15(22): 5309. CrossRef - Proliferative hepatocellular carcinomas in cirrhosis: patient outcomes of LI-RADS category 4/5 and category M
Subin Heo, Hyo Jeong Kang, Sang Hyun Choi, Sehee Kim, Youngeun Yoo, Won-Mook Choi, So Yeon Kim, Seung Soo Lee
European Radiology.2023;[Epub] CrossRef - Cellular heterogeneity and plasticity in liver cancer
Lo-Kong Chan, Yu-Man Tsui, Daniel Wai-Hung Ho, Irene Oi-Lin Ng
Seminars in Cancer Biology.2022; 82: 134. CrossRef - MRI features of histologic subtypes of hepatocellular carcinoma: correlation with histologic, genetic, and molecular biologic classification
Ja Kyung Yoon, Jin-Young Choi, Hyungjin Rhee, Young Nyun Park
European Radiology.2022; 32(8): 5119. CrossRef - Variant Hepatocellular Carcinoma Subtypes According to the 2019 WHO Classification: An Imaging-Focused Review
Liang Meng Loy, Hsien Min Low, Jin-Young Choi, Hyungjin Rhee, Chin Fong Wong, Cher Heng Tan
American Journal of Roentgenology.2022; 219(2): 212. CrossRef - Paraneoplastic syndromes in hepatocellular carcinoma: a review
Yuki Ong, Cheong Wei Terence Huey, Vishalkumar Girishchandra Shelat
Expert Review of Gastroenterology & Hepatology.2022; 16(5): 449. CrossRef - Morphomolecular Classification Update on Hepatocellular Adenoma, Hepatocellular Carcinoma, and Intrahepatic Cholangiocarcinoma
Venkata S. Katabathina, Lokesh Khanna, Venkateswar R. Surabhi, Marta Minervini, Krishna Shanbhogue, Anil K. Dasyam, Srinivasa R. Prasad
RadioGraphics.2022; 42(5): 1338. CrossRef - 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma
Clinical and Molecular Hepatology.2022; 28(4): 583. CrossRef - 2022 KLCA-NCC Korea Practice Guidelines for the Management of Hepatocellular Carcinoma
Korean Journal of Radiology.2022; 23(12): 1126. CrossRef - Histomorphological Subtypes of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma: Review and Update
Yoon Jung Hwang, Haeryoung Kim
AJSP: Reviews and Reports.2022; 27(6): 234. CrossRef - Pediatric Primary Hepatic Tumors: Diagnostic Considerations
Bryony Lucas, Sanjita Ravishankar, Irina Pateva
Diagnostics.2021; 11(2): 333. CrossRef - A Case of Lymphocyte-Rich Hepatocellular Carcinoma in a Patient Who Was Treated for Colon Cancer
Jae Won Song, Ho Soo Chun, Jae Seung Lee, Hye Won Lee, Beom Kyung Kim, Seung Up Kim, Jun Yong Park, Sang Hoon Ahn, Young Nyun Park, Dai Hoon Han, Do Young Kim
Journal of Liver Cancer.2021; 21(1): 69. CrossRef - HCC You Cannot See
Vaishnavi Boppana, Sakshi Sahni, Joseph Glass, Christopher Chang, Denis M McCarthy
Digestive Diseases and Sciences.2021; 66(7): 2185. CrossRef - An update on subtypes of hepatocellular carcinoma: From morphology to molecular
Monika Vyas, Dhanpat Jain
Indian Journal of Pathology and Microbiology.2021; 64(5): 112. CrossRef - HCC: role of pre- and post-treatment tumor biology in driving adverse outcomes and rare responses to therapy
Sandeep Arora, Roberta Catania, Amir Borhani, Natally Horvat, Kathryn Fowler, Carla Harmath
Abdominal Radiology.2021; 46(8): 3686. CrossRef - Gadoxetate-enhanced MRI Features of Proliferative Hepatocellular Carcinoma Are Prognostic after Surgery
Hyo-Jin Kang, Haeryoung Kim, Dong Ho Lee, Bo Yun Hur, Yoon Jung Hwang, Kyung-Suk Suh, Joon Koo Han
Radiology.2021; 300(3): 572. CrossRef - Radiologic Diagnosis of Hepatocellular Carcinoma
Woo Kyoung Jeong
The Korean Journal of Gastroenterology.2021; 78(5): 261. CrossRef - Update on Hepatocellular Carcinoma: a Brief Review from Pathologist Standpoint
Nese Karadag Soylu
Journal of Gastrointestinal Cancer.2020; 51(4): 1176. CrossRef
- Computational pathology: A survey review and the way forward

- Deciphering and Reversing Immunosuppressive Cells in the Treatment of Hepatocellular Carcinoma
- Su Jong Yu, Tim F. Greten
- J Liver Cancer. 2020;20(1):1-16. Published online March 31, 2020
- DOI: https://doi.org/10.17998/jlc.20.1.1
- 6,786 Views
- 191 Downloads
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF - Use of immune checkpoint inhibitors (ICIs) in hepatocellular carcinoma (HCC) has been partially successful. However, most HCC patients do not respond to immunotherapy. HCC has been shown to induce several immune suppressor mechanisms in patients. These suppressor mechanisms include involvement of myeloid-derived suppressor cells, regulatory T-cells, functionally impaired dendritic cells (DCs), neutrophils, monocytes, and tumor associated macrophages. The accumulation of immunosuppressive cells may lead to an immunosuppressive tumor microenvironment as well as the dense fibrotic stroma which may contribute to immune tolerance. Our laboratory has been investigating different cellular mechanisms of immune suppression in HCC patients. In vitro as well as in vivo studies have demonstrated that abrogation of the suppressor cells enhances or unmasks tumor-specific antitumor immune responses. Two or three effective systemic therapies including ICIs and/or molecular targeted therapies and the addition of innovative combination therapies targeting immune suppressor cells may lead to increased immune recognition with a greater tumor response. We reviewed the literature for the latest research on immune suppressor cells in HCC, and here we provide a comprehensive summary of the recent studies in this field.
-
Citations
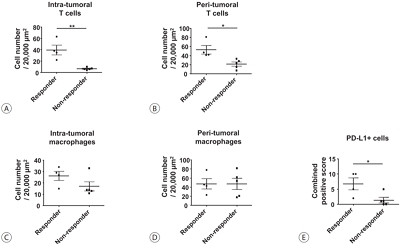
Citations to this article as recorded by- Higher Number of Tumor-Infiltrating PD-L1+ Cells Is Related to Better Response to Multikinase Inhibitors in Hepatocellular Carcinoma
Ji Won Han, Ji Hoon Kim, Dong Hyun Kim, Jeong Won Jang, Si Hyun Bae, Jong Young Choi, Seung Kew Yoon, Jaegyoon Ahn, Hyun Yang, Pil Soo Sung
Diagnostics.2023; 13(8): 1453. CrossRef - Immune checkpoint inhibitors in HCC: Cellular, molecular and systemic data
Uasim Harkus, Miriam Wankell, Pranavan Palamuthusingam, Craig McFarlane, Lionel Hebbard
Seminars in Cancer Biology.2022; 86: 799. CrossRef - Crosstalk between tumor-associated macrophages and neighboring cells in hepatocellular carcinoma
Pil Soo Sung
Clinical and Molecular Hepatology.2022; 28(3): 333. CrossRef
- Higher Number of Tumor-Infiltrating PD-L1+ Cells Is Related to Better Response to Multikinase Inhibitors in Hepatocellular Carcinoma

- The Genomic Landscape and Its Clinical Implications in Hepatocellular Carcinoma
- Sun Young Yim, Ju-Seog Lee
- J Liver Cancer. 2019;19(2):97-107. Published online September 30, 2019
- DOI: https://doi.org/10.17998/jlc.19.2.97

- 6,459 Views
- 253 Downloads
- 7 Citations
-
 Abstract
Abstract
 PDF
PDF - The pathogenesis of hepatocellular carcinoma (HCC) is a complex process. During the last decade, advances in genomic technologies enabled delineation of the genomic landscape of HCC, resulting in the identification of the common underlying molecular alterations. The tumor microenvironment, regulated by inflammatory cells, including cancer cells, stromal tissues, and the surrounding extracellular matrix, has been extensively studied using molecular data. The integration of molecular, immunological, histopathological, and clinical findings has provided clues to uncover predictive biomarkers to enhance responses to novel therapies. Herein, we provide an overview of the current HCC genomic landscape, previously identified gene signatures that are used routinely to predict prognosis, and an immune-specific class of HCC. Since biomarker-driven treatment is still an unmet need in HCC management, translation of these discoveries into clinical practice will lead to personalized therapies and improve patient care, especially in the era of targeted and immunotherapies.
-
Citations
Citations to this article as recorded by- Comprehensive clinicopathologic study of alpha fetoprotein‐expression in a large cohort of patients with hepatocellular carcinoma
Dirk Andreas Ridder, Arndt Weinmann, Mario Schindeldecker, Lana Louisa Urbansky, Kristina Berndt, Tiemo Sven Gerber, Hauke Lang, Johannes Lotz, Karl J. Lackner, Wilfried Roth, Beate Katharina Straub
International Journal of Cancer.2022; 150(6): 1053. CrossRef - Two distinct stem cell‐like subtypes of hepatocellular carcinoma with clinical significance and their therapeutic potentials
Sung Hwan Lee, Yun Seong Jeong, Sunyoung Lee, Bo Hwa Sohn, Ho Kyoung Hwang, Gi Hong Choi, Chang Moo Kang, Jin Sub Choi, Woo Jung Lee, Jae‐Ho Cheong, Hee Jin Jang, Ahmed Kaseb, Lewis Roberts, Sun Young Yim, Yun Shin Chun, Ju‐Seog Lee
Cancer Communications.2022; 42(2): 179. CrossRef - Activated TAZ induces liver cancer in collaboration with EGFR/HER2 signaling pathways
Hyuk Moon, Hyunjung Park, Min Jee Chae, Hye Jin Choi, Do Young Kim, Simon Weonsang Ro
BMC Cancer.2022;[Epub] CrossRef - Tumor aggressiveness is independent of radiation quality in murine hepatocellular carcinoma and mammary tumor models
Eshwar B. Udho, Shane M. Huebner, Dawn M. Albrecht, Kristina A. Matkowskyj, Linda Clipson, Catigan A. Hedican, Rachel Koth, Santina M. Snow, Emily L. Eberhardt, Devon Miller, Rachel Van Doorn, Genti Gjyzeli, Erin K. Spengler, Douglas R. Storts, Douglas H.
International Journal of Radiation Biology.2021; 97(8): 1140. CrossRef - Infiltrative hepatocellular carcinoma with multiple lung metastasis completely cured using nivolumab: a case report
Ji Eun Han, Hyo Jung Cho, Soon Sun Kim, Jae Youn Cheong
Journal of Liver Cancer.2021; 21(2): 169. CrossRef - Update on Hepatocellular Carcinoma: a Brief Review from Pathologist Standpoint
Nese Karadag Soylu
Journal of Gastrointestinal Cancer.2020; 51(4): 1176. CrossRef - Hepatocellular carcinoma: new provisions of the WHO classification, 5th edition, 2019
E.M. Nepomnyashchaya, A.V. Shaposhnikov, E.A. Yurieva
Arkhiv patologii.2020; 82(6): 36. CrossRef
- Comprehensive clinicopathologic study of alpha fetoprotein‐expression in a large cohort of patients with hepatocellular carcinoma

- Contrast-enhanced Ultrasonography: The Third Modality for Differentiation of Liver Mass
- Min Kyu Kang, Moon Young Kim, Seong Hee Kang, Soon Koo Baik
- J Liver Cancer. 2019;19(2):91-96. Published online September 30, 2019
- DOI: https://doi.org/10.17998/jlc.19.2.91
- 4,655 Views
- 96 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
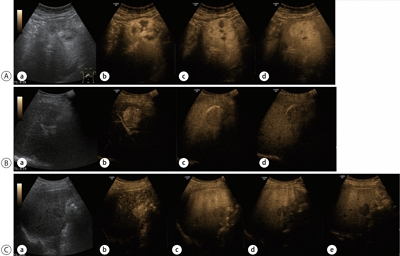
PDF - Contrast-enhanced ultrasonography (CEUS) using microbubble ultrasonography contrast agent can show the vascular structure and unique contrast enhancement patterns of focal liver lesions, including hepatocellular carcinoma (HCC). CEUS shows three phases, similar to a vascular pattern on computer tomography (CT), and typical arterial enhancement and portal or late phase washout in HCC. CEUS can show real-time images without nephrotoxicity or radiation hazard and can be used as guidance for loco-regional treatment and estimation of treatment response of HCC. In addition, some data recently revealed the usefulness of CEUS in the early estimation of response to anti-cancer pharmacological (i.e., sorafenib) therapy in advanced HCC. Although CEUS has limitations in clinical practice and more investigation is needed for its validation, it is recommended as a main diagnostic modality in a few major clinical practice guidelines for HCC. Thus, greater understanding of CEUS is necessary to extend its application in real practice for diagnosis and management of diseases.
-
Citations
Citations to this article as recorded by- Perfluorobutane-Enhanced Ultrasound for Characterization of Hepatocellular Carcinoma From Non-hepatocellular Malignancies or Benignancy: Comparison of Imaging Acquisition Methods
Seungchul Han, Se Woo Kim, Sungeun Park, Jeong Hee Yoon, Hyo-Jin Kang, Jeongin Yoo, Ijin Joo, Jae Seok Bae, Jeong Min Lee
Ultrasound in Medicine & Biology.2023; 49(10): 2256. CrossRef
- Perfluorobutane-Enhanced Ultrasound for Characterization of Hepatocellular Carcinoma From Non-hepatocellular Malignancies or Benignancy: Comparison of Imaging Acquisition Methods

- Liver Transplantation in Mixed Hepatocellular Carcinoma and Cholangiocarcinoma
- Jong Man Kim
- J Liver Cancer. 2019;19(2):85-90. Published online September 30, 2019
- DOI: https://doi.org/10.17998/jlc.19.2.85
- 3,831 Views
- 89 Downloads
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF - Mixed hepatocellular carcinoma and cholangiocarcinoma (HCC-CC) are rare tumors, and the risk factors associated with them are not well understood yet. Moreover, the diagnosis of mixed HCC-CC can be complicated due to the difficulty in distinguishing mixed HCC-CC from HCC and intrahepatic CCC on radiological images. Serum tumor markers are useful when the radiological images are inconclusive. It remains unclear whether the prognosis of mixed HCC-CC differs from that of HCC. However, several studies have reported that the tumor recurrence and patient survival rates of mixed HCC-CC were similar to those of HCC after liver transplantation (LT) and liver resection. In this paper, we report that LT in patients with mixed HCC-CC achieves outcomes which are similar to those seen in LT for HCC. Therefore, the diagnosis of mixed HCC-CC should not be considered as a contraindication for LT.
-
Citations
Citations to this article as recorded by- Liver transplantation for combined hepatocellular carcinoma and cholangiocarcinoma: A multicenter study
Jongman Kim, Dong-Jin Joo, Shin Hwang, Jeong-Moo Lee, Je-Ho Ryu, Yang-Won Nah, Dong-Sik Kim, Doo-Jin Kim, Young-Kyoung You, Hee-Chul Yu
World Journal of Gastrointestinal Surgery.2023; 15(7): 1340. CrossRef - The Role of Immunosuppression for Recurrent Cholangiocellular Carcinoma after Liver Transplantation
Safak Gül-Klein, Paulina Schmitz, Wenzel Schöning, Robert Öllinger, Georg Lurje, Sven Jonas, Deniz Uluk, Uwe Pelzer, Frank Tacke, Moritz Schmelzle, Johann Pratschke, Ramin Raul Ossami Saidy, Dennis Eurich
Cancers.2022; 14(12): 2890. CrossRef
- Liver transplantation for combined hepatocellular carcinoma and cholangiocarcinoma: A multicenter study

Case Reports
- Early Onset Polymorphic Post-transplant Lymphoproliferative Disease Mimicking a Solitary Necrotizing Abscess in a Graft Liver
- Pil Soo Sung, Jaejun Lee, Joon Lee, Hee Chul Nam, Si Hyun Bae, Seung Kew Yoon
- J Liver Cancer. 2019;19(2):165-170. Published online September 30, 2019
- DOI: https://doi.org/10.17998/jlc.19.2.165

- 4,849 Views
- 74 Downloads
-
 Abstract
Abstract
 PDF
PDF - Although post-transplantation lymphoproliferative disease (PTLD) after liver transplantation is very rare, its prognosis is worse than that of PTLD following other types of solid organ transplantation. Here, we report a rare case of early onset polymorphic PTLD in a graft liver occurring five months after deceased-donor liver transplantation due to hepatocellular carcinoma and hepatitis C virus infection. Initially, findings from contrast-enhanced magnetic resonance imaging mistakenly suspected the lesion was a necrotizing abscess with central necrosis. However, 18F-fluorodeoxyglucose positron emission tomography and biopsy findings confirmed an Epstein-Barr virus (EBV)-associated, B cell type polymorphic PTLD with central necrosis. Our case suggests regular monitoring of EBV serologic status for liver transplant recipients who were initially in an EBV seronegative state. Although early-onset PTLD is very rare after liver transplantation, PTLD should be suspected when recipients show the seroconversion for EBV proteins and the development of new tumors with various clinical presentations.

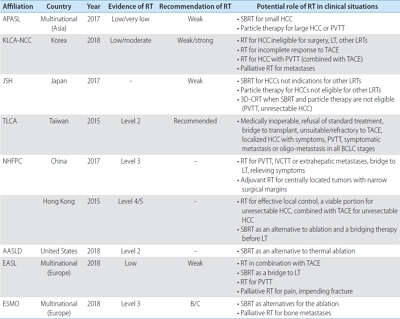
- Hepatocellular Carcinoma with Segmental Portal Vein Invasion Exhibiting a Complete Response after Transarterial Radioembolization
- Jun Sik Yoon, Su Jong Yu, Yun Bin Lee, Eun Ju Cho, Jeong-Hoon Lee, Yoon Jun Kim, Jung-Hwan Yoon
- J Liver Cancer. 2019;19(2):159-164. Published online September 30, 2019
- DOI: https://doi.org/10.17998/jlc.19.2.159

- 5,303 Views
- 73 Downloads
-
 Abstract
Abstract
 PDF
PDF - The treatment options available for patients with hepatocellular carcinoma (HCC) with portal vein invasion (PVI) include sorafenib, transarterial radioembolization (TARE), radiation therapy (RT), transarterial chemoembolization with RT, and proton beam irradiation. Herein, we present a case of HCC with segmental PVI that was managed via TARE. The patient had a 4 cm HCC that invaded the segment VIII portal vein branch without extrahepatic spread. Liver function was Child-Pugh grade A, and performance status was good. TARE was performed without any adverse events, and a radiological complete response (CR) was achieved. Thereafter, the patient was followed-up every 3-6 months without any further treatment, and the CR was maintained for >3 years. Therefore, TARE may be a useful alternative therapeutic option for patients with HCC exhibiting segmental PVI.

- Sorafenib-induced Pancreatic Pseudocyst in a Patient with Advanced Hepatocellular Carcinoma: a Rare Adverse Event
- Dae-ha Kim, Minkoo Kim, Hyung Joon Yim, Sang Jun Suh, Young Kul Jung
- J Liver Cancer. 2019;19(2):154-158. Published online September 30, 2019
- DOI: https://doi.org/10.17998/jlc.19.2.154
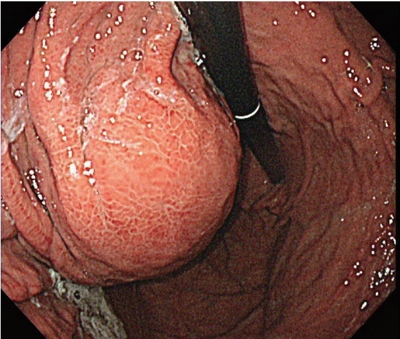
- 3,437 Views
- 47 Downloads
-
 Abstract
Abstract
 PDF
PDF - A 54-year old man diagnosed with advanced hepatocellular carcinoma began treatment with sorafenib. After 3 weeks of treatment, he complained of abdominal pain and nausea. Abdominal sonography showed multiple hepatic lesions only. Serum amylase and lipase levels were 35 U/L and 191 U/L, respectively. The patient was diagnosed with sorafenib-induced acute pancreatitis. After 10 days of discontinuing sorafenib he still complained of nausea and loss of appetite. Esophagogastroduodenoscopy showed a large bulging lesion, which was suspected to cause extrinsic compression on the high body of the gastric anterior wall. Computed tomography scan revealed a cystic lesion, 8.3 cm in size, in the pancreatic tail, suggesting a pancreatic pseudocyst. After the withdrawal of sorafenib, systemic chemotherapy with Adriamycin and cisplatin was administered. Four months after the discontinuation of sorafenib, the size of the pancreatic pseudocyst decreased from 8.3 cm to 3 cm. The patient's symptoms were also relieved.

- Long-term Disease-free Survival after Trimodality Treatment of Recurrent Hepatocellular Carcinoma Involving the Inferior Vena Cava and Right Atrium
- Sunmin Park, Won Sup Yoon, Hyung Joon Yim, Chai Hong Rim
- J Liver Cancer. 2019;19(2):149-153. Published online September 30, 2019
- DOI: https://doi.org/10.17998/jlc.19.2.149
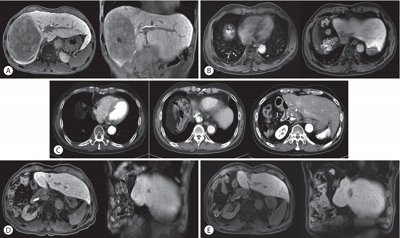
- 3,564 Views
- 73 Downloads
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Hepatocellular carcinoma (HCC) involving the inferior vena cava (IVC) and/or right atrium (RA) is a rare and intractable disease. A standard treatment has not been established yet, owing to the rarity of disease and difficulties in the therapeutic treatment. Herein, we report the case of a patient who had recurrent HCC (after a prior lobectomy) involving both IVC and RA and underwent multimodality treatments including external beam radiotherapy and transarterial chemotherapy, followed by sorafenib treatment. The disease was well controlled with local treatments and sustained for 7 years until last follow-up after the systemic treatments. Our case shows a possibility of long-term survival for patients affected by HCC involving IVC and/or RA, after a rigorous multimodality treatment strategy.

- Malignant Hepatic Solitary Fibrous Tumor
- Hong Il Kim, Seok Kyung In, Hyung Suk Yi, Min Jeong Lee, Hyo Young Kim
- J Liver Cancer. 2019;19(2):143-148. Published online September 30, 2019
- DOI: https://doi.org/10.17998/jlc.19.2.143

- 3,563 Views
- 51 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
PDF - Hepatic solitary fibrous tumors (SFTs) are mostly benign and rare because of information regarding the clinical symptoms, treatment, and prognosis of their malignant forms is currently lacking. A literature review concerning malignant SFTs revealed that there were a few cases where patients experienced abdominal right upper quadrant (RUQ) pain as their first clinical symptom, and metastases were found after being diagnosed with hepatic SFT. Here, we report a patient who was previously healthy without any clinical symptoms such as RUQ pain or weight loss, but had the appearance of a metastatic mass as the first clinical presentation before a primary hepatic SFT was detected.
-
Citations
Citations to this article as recorded by- A Case of Hepatic Malignant Solitary Fibrous Tumor: A Case Report and Review of the Literature
Zhiyan Fu, Evita B. Henderson-Jackson, Barbara A. Centeno, Gregory Y. Lauwers, Mihaela Druta, Daniel A. Anaya, Yukihiro Nakanishi, Samir Sami Amr
Case Reports in Pathology.2023; 2023: 1. CrossRef
- A Case of Hepatic Malignant Solitary Fibrous Tumor: A Case Report and Review of the Literature

- Radiation-induced Myositis after Proton Beam Therapy to Huge Hepatocellular Carcinoma
- Jihye Kim, Gyu Sang Yoo, Dong Hyun Sinn, Hee Chul Park, Kwang Cheol Koh
- J Liver Cancer. 2019;19(2):136-142. Published online September 30, 2019
- DOI: https://doi.org/10.17998/jlc.19.2.136
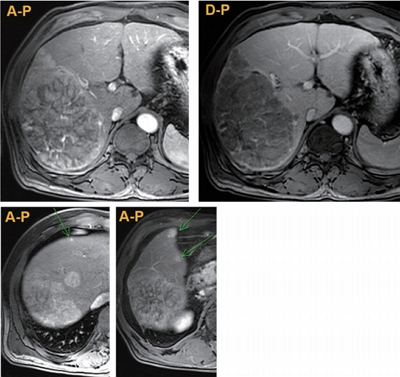
- 6,151 Views
- 119 Downloads
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF - Proton beam therapy (PBT) is one of the advances in radiotherapy techniques, which enables dose escalation with lower probability of radiation-induced liver or gastrointestinal injuries. However, the chest wall proximal to the tumor can be affected by high dose irradiation. Here, we report on a 58-year-old male patient who presented with huge hepatocellular carcinoma, received treatment with transarterial chemoembolization and PBT, and developed severe chest wall pain due to radiation-induced myositis. The patient’s symptoms were controlled by oral steroids.
-
Citations
Citations to this article as recorded by- Pectoralis Major Radiation Myonecrosis After Lung Stereotactic Body Radiation Therapy
Jason Gurewitz, Anand Mahadevan, Benjamin T. Cooper
Practical Radiation Oncology.2023;[Epub] CrossRef - Current role of proton beam therapy in patients with hepatocellular carcinoma
Gyu Sang Yoo, Jeong Il Yu, Hee Chul Park
International Journal of Gastrointestinal Intervention.2021; 10(4): 175. CrossRef
- Pectoralis Major Radiation Myonecrosis After Lung Stereotactic Body Radiation Therapy

Original Articles
- Tenofovir and Entecavir Have Similar Renal Adverse Events on Hepatocellular Carcinoma Patients Treated with Transarterial Chemoembolization
- Young Youn Cho, Young Hwan Choi, Su Jong Yu, Eun Ju Cho, Jeong-Hoon Lee, Yoon Jun Kim, Jung-Hwan Yoon
- J Liver Cancer. 2019;19(2):128-135. Published online September 30, 2019
- DOI: https://doi.org/10.17998/jlc.19.2.128
- 3,808 Views
- 47 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
PDF - Background/Aim
s: Tenofovir disoproxil fumarate (TDF) is potentially nephrotoxic in chronic hepatitis B patients. Hepatocellular carcinoma (HCC) patients treated using transarterial chemoembolization (TACE) are at an increased risk of renal injury. The aim of this study was to determine whether TDF is associated with more renal adverse events than entecavir (ETV) in HCC patients treated with TACE.
Methods
In this retrospective single-center study, we selected 53 HCC patients who were treated with TDF from January 2012 to July 2013 and had their first TACE procedure in the same period. These patients were matched by age and sex to patients treated with ETV.
Results
There were no significant differences in baseline characteristics, including HCC factors, and nephrotoxic drug use, between the two groups. The median follow-up period was 17.0 and 20.0 months for the TDF and ETV groups, respectively. There was no difference during the follow-up period between the TDF and ETV groups in the increase in creatinine over 0.5 mg/dL (17.0% and 17.0%, P=1.00, respectively) and the decrease in eGFR over 25% (43.4% and 41.5%, P=0.84, respectively). Multivariate analysis revealed that Child-Pugh class over B (hazard ratio [HR], 7.30; 95% confidence interval [CI] 2.79-19.10; P<0.01) was associated with increase in creatinine, and Child-Pugh class over B (HR, 82.74; 95% CI 12.31-555.83; P<0.01) and Barcelona-Clinic Liver Cancer stage over B (HR, 14.93; 95% CI 1.60-139.51; P=0.02) were associated with decrease in eGFR.
Conclusions
TDF has comparable safety to that of ETV for HCC patients undergoing TACE. -
Citations
Citations to this article as recorded by- Big Data Information under Proportional Hazard Mathematical Model in Analysis of Hepatitis B Virus Infection Data of Patients with Interventional Liver Cancer through Antiviral Therapy of Entecavir
Yichi Zhang, Shuai Zhao, Han Ding, Xiaoling Song, Huijie Miao, Xuya Cui, Jian Wang, Bing Han, Enas Abdulhay
Journal of Healthcare Engineering.2021; 2021: 1. CrossRef
- Big Data Information under Proportional Hazard Mathematical Model in Analysis of Hepatitis B Virus Infection Data of Patients with Interventional Liver Cancer through Antiviral Therapy of Entecavir

- An Analysis for Survival Predictors for Patients with Hepatocellular Carcinoma Who Failed to Sorafenib Treatment in Pre-regorafenib Era
- Chan Uk Lee, Young-Sun Lee, Ji Hoon Kim, Minjin Lee, Sehwa Kim, Young Kul Jung, Yeon Seok Seo, Hyung Joon Yim, Jong Eun Yeon, Kwan Soo Byun
- J Liver Cancer. 2019;19(2):117-127. Published online September 30, 2019
- DOI: https://doi.org/10.17998/jlc.19.2.117

- 4,206 Views
- 64 Downloads
-
 Abstract
Abstract
 PDF
PDF - Background/Aim
s: Sorafenib is the standard treatment for patients with advanced hepatocellular carcinoma (HCC). We aimed to investigate the prognosis predictors and the role of second-line cytotoxic systemic chemotherapy (CSC) in patients with advanced HCC after sorafenib discontinuation in the pre-regorafenib era.
Methods
From 2007 to 2015 in the pre-regorafenib era, the medical records of 166 HCC patients, who had permanently discontinued sorafenib, were retrospectively reviewed. For further analysis of survival factors after sorafenib treatment failure, we compared the survival of patients who had maintained liver function after second-line treatment with the best supportive care (BSC) group and selective BSC (SBSC) group.
Results
After discontinuation of sorafenib, median overall survival (OS) was 2.8 (1.9-3.7) months. The OS in patients who discontinued sorafenib due to adverse effect, progression, and poor clinical condition were 5.5 (2.4-8.6), 5.5 (2.2-8.9), and 0.9 (0.5-1.3) months, respectively (P<0.001). The independent predictive factors of survival after sorafenib failure were serum level of bilirubin and albumin, α-fetoprotein, discontinuation cause, and second-line CSC. In comparison with survival between second-line CSC and BSC group, the CSC group showed better survival outcome compared to the BSC group (10.6 vs. 1.6 months, P<0.001) and SBSC group (10.6 vs. 4.2 months, P=0.023).
Conclusions
The survival after sorafenib failure in patients who discontinued sorafenib due to progression and adverse effects was significantly better than in those who discontinued treatment due to clinical deterioration. In the pre-regorafenib era, patients who received second-line CSC showed better survival than those who received only supportive care after sorafenib failure.


 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION

 First
First Prev
Prev



 Follow JLC on Twitter
Follow JLC on Twitter